Surge in Syphilis Cases Leads Some Providers to Ration Penicillin
When Stephen Miller left his primary care practice to work in public health a little under two years ago, he said, he was shocked by how many cases of syphilis the clinic was treating.
For decades, rates of the sexually transmitted infection were low. But the Hamilton County Health Department in Chattanooga — a midsize city surrounded by national forests and nestled into the Appalachian foothills of Tennessee — was seeing several syphilis patients a day, Miller said. A nurse who had worked at the clinic for decades told Miller the wave of patients was a radical change from the norm.
What Miller observed in Chattanooga is reflective of a trend that is raising alarm bells for health departments across the country.
Nationwide, syphilis rates are at a 70-year high. The Centers for Disease Control and Prevention said Jan. 30 that 207,255 cases were reported in 2022, continuing a steep increase over five years. Between 2018 and 2022, syphilis rates rose about 80%. The epidemic of sexually transmitted infections — especially syphilis — is “out of control,” said the National Coalition of STD Directors.
The surge has been even more pronounced in Tennessee, where infection rates for the first two stages of syphilis grew 86% between 2017 and 2021.
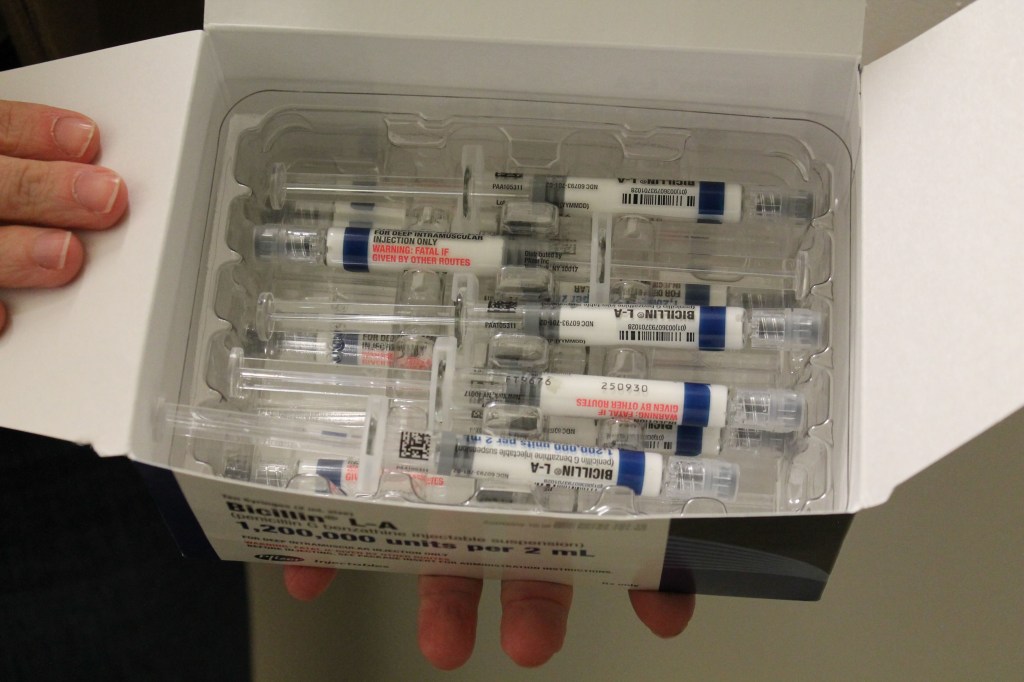
But this already difficult situation was complicated last spring by a shortage of a specific penicillin injection that is the go-to treatment for syphilis. The ongoing shortage is so severe that public health agencies have recommended that providers ration the drug — prioritizing pregnant patients, since it is the only syphilis treatment considered safe for them. Congenital syphilis, which happens when the mom spreads the disease to the fetus, can cause birth defects, miscarriages, and stillbirths.
Across the country, 3,755 cases of congenital syphilis were reported to the CDC in 2022 — that’s 10 times as high as the number a decade before, the recent data shows. Of those cases, 231 resulted in stillbirth and 51 led to infant death. The number of cases in babies swelled by 183% between 2018 and 2022.
“Lack of timely testing and adequate treatment during pregnancy contributed to 88% of cases of congenital syphilis,” said a report from the CDC released in November. “Testing and treatment gaps were present in the majority of cases across all races, ethnicities, and U.S. Census Bureau regions.”
Hamilton County’s syphilis rates have mirrored the national trend, with an increase in cases for all groups, including infants.
In November, the maternal and infant health advocacy organization March of Dimes released its annual report on states’ health outcomes. It found that, nationwide, about 15.5% of pregnant people received care beginning in the fifth month of pregnancy or later — or attended fewer than half the recommended prenatal visits. In Tennessee, the rate was even worse, 17.4%.
But Miller said even those who attend every recommended appointment can run into problems because providers are required to test for syphilis only at the beginning of a pregnancy. The idea is that if you test a few weeks before birth, there is time to treat the infection.
However, that recommendation hinges on whether the provider suspects the patient was exposed to the bacterium that causes syphilis, which may not be obvious for people who say their relationships are monogamous.
“What we found is, a lot of times their partner was not as monogamous, and they were bringing it into the relationship,” Miller said.
Even if the patient tested negative initially, they may have contracted syphilis later in pregnancy, when testing for the disease is not routine, he said.
Two antibiotics are used to treat syphilis, the injectable penicillin and an oral drug called doxycycline.
Patients allergic to penicillin are often prescribed the oral antibiotic. But the World Health Organization strongly advises pregnant patients to avoid doxycycline because it can cause severe bone and teeth deformities in the infant.
As a result, pregnant syphilis patients are often given penicillin, even when they’re allergic, using a technique called desensitization, said Mark Turrentine, a Houston OB-GYN. Patients are given low doses in a hospital setting to help their bodies get used to the drug and to check for a severe reaction. The penicillin shot is a one-and-done technique, unlike an antibiotic, which requires sticking to a two-week regimen.
“It’s tough to take a medication for a long period of time,” Turrentine said. The single injection can provide patients and their clinicians peace of mind. “If they don’t come back for whatever reason, you’re not worried about it,” he said.
The Metro Public Health Department in Nashville, Tennessee, began giving all nonpregnant adults with syphilis the oral antibiotic in July, said Laura Varnier, nursing and clinical director.
Turrentine said he started seeing advisories about the injectable penicillin shortage in April, around the time the antibiotic amoxicillin became difficult to find and physicians were using penicillin as a substitute, potentially precipitating the shortage, he said.
The rise in syphilis has created demand for the injection that manufacturer Pfizer can’t keep up with, according to the American Society of Health-System Pharmacists. “There is insufficient supply for usual ordering,” the ASHP said in a memo.
Even though penicillin has been around a long time, manufacturing it is difficult, largely because so many people are allergic, said Erin Fox, associate chief pharmacy officer for the University of Utah health system and an adjunct professor at the university, who studies drug shortages.
“That means you can’t make other drugs on that manufacturing line,” she said. Only major manufacturers like Pfizer have the resources to build and operate such a specialized, cordoned-off facility. “It’s not necessarily efficient — or necessarily profitable,” Fox said.
In a statement, Pfizer confirmed the amoxicillin shortage and surge in syphilis increased demand for injectable penicillin by about 70%. Representatives said the company invested $38 million in the facility that produces this form of penicillin, hiring more staff and expanding the production line.
“This ramp up will take some time to be felt in the market, as product cycle time is 3-6 months from when product is manufactured to when it is available to be released to customers,” the statement reads. The company estimated the shortage would be significantly alleviated by spring.
In the meantime, Miller said, his clinic in Chattanooga is continuing to strategize. Each dose of injectable penicillin can cost hundreds of dollars. Plus, it has to be placed in cold storage, and it expires after 48 months.
Even with the dramatic increase in cases, syphilis is still relatively rare. More than 7 million people live in Tennessee, and in 2019, providers statewide reported 683 cases of syphilis.
Health departments like Miller’s treat the bulk of syphilis patients. Many patients are sent by their provider to the health department, which works with contact tracers to identify and notify sexual partners who might be affected and tests patients for other sexually transmitted infections, including HIV.
“When you diagnose in the office, think of it as just seeing the tip of the iceberg,” Miller said. “You need a team of individuals to be able to explore and look at the rest of the iceberg.”
This story is part of a partnership that includes WPLN, NPR, and KFF Health News.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 5 months ago
Pharmaceuticals, Public Health, Rural Health, States, CDC, Sexual Health, Tennessee
KFF Health News' 'What the Health?': Health Enters the Presidential Race
The Host
Julie Rovner
KFF Health News
Julie Rovner is chief Washington correspondent and host of KFF Health News’ weekly health policy news podcast, “What the Health?” A noted expert on health policy issues, Julie is the author of the critically praised reference book “Health Care Politics and Policy A to Z,” now in its third edition.
Based on the results of the first-in-the-nation primary in New Hampshire, it appears more likely than ever before that the 2024 presidential election will be a rerun of 2020: Joe Biden versus Donald Trump. And health is shaping up to be a key issue.
Trump is vowing — again — to repeal the Affordable Care Act, which is even more popular than it was when Republicans failed to muster the congressional votes to kill it in 2017. Biden is doubling down on support for contraception and abortion rights.
And both are expected to highlight efforts to rein in the cost of prescription drugs.
This week’s panelists are Julie Rovner of KFF Health News, Alice Miranda Ollstein of Politico, Anna Edney of Bloomberg News, and Jessie Hellmann of CQ Roll Call.
Panelists
Alice Miranda Ollstein
Politico
Anna Edney
Bloomberg
Jessie Hellmann
CQ Roll Call
Among the takeaways from this week’s episode:
- Trump had a strong showing in the New Hampshire GOP primary. But Biden may be gathering momentum himself from an unexpected source: Drug industry lawsuits challenging his administration’s Medicare price negotiation plan could draw attention to Biden’s efforts to combat rising prescription drug prices, a major pocketbook issue for many voters.
- Biden’s drug pricing efforts also include using the government’s so-called march-in rights on pharmaceuticals, which could allow the government to lower prices on certain drugs — it’s unclear which ones. Meanwhile, Sen. Bernie Sanders of Vermont is calling on his committee to subpoena the CEOS of two drugmakers in the latest example of lawmakers summoning Big Pharma executives to the Hill to answer for high prices.
- More than a year after the Supreme Court overturned the constitutional right to an abortion, abortion opponents gathered in Washington, D.C., for the March for Life rally, looking now to continue to advance their priorities under a future conservative presidency.
- One avenue that abortion opponents are eying is the 19th-century Comstock Act, which could not only prohibit the mailing of abortion pills to patients, but also prevent them from being mailed to clinics and medical facilities. Considering the abortion pill is now used in more than half of abortions nationwide, it would amount to a fairly sweeping ban.
- And state legislators continue to push more restrictive abortion laws, targeting care for minors and rape exceptions in particular. The ongoing quest to winnow access to the procedure amid public reservations reflected in polling and ballot initiatives highlights that, for at least some abortion opponents, fetuses are framed as an oppressed minority whose rights should not be subject to a majority vote.
Also this week, Rovner interviews Sarah Somers, legal director of the National Health Law Program, about the potential effects on federal health programs if the Supreme Court overturns a 40-year-old precedent established in the case Chevron USA v. Natural Resources Defense Council.
Plus, for “extra credit,” the panelists suggest health policy stories they read this week that they think you should read, too:
Julie Rovner: Health Affairs’ “‘Housing First’ Increased Psychiatric Care Office Visits and Prescriptions While Reducing Emergency Visits,” by Devlin Hanson and Sarah Gillespie.
Alice Miranda Ollstein: Stat’s “The White House Has a Pharmacy — And It Was a Mess, a New Investigation Found,” by Brittany Trang.
Anna Edney: The New Yorker’s “What Would It Mean for Scientists to Listen to Patients?” by Rachael Bedard.
Jessie Hellmann: North Carolina Health News’ “Congenital Syphilis — An Ancient Scourge — Claimed the Lives of Eight NC Babies Last Year,” by Jennifer Fernandez.
Also mentioned on this week’s podcast:
Stat’s “Pharma’s Attack on Medicare Drug Price Negotiation Might Benefit Biden,” by John Wilkerson.
click to open the transcript
Transcript: Health Enters the Presidential Race
KFF Health News’ ‘What the Health?’Episode Title: Health Enters the Presidential RaceEpisode Number: 331Published: Jan. 25, 2024
[Editor’s note: This transcript was generated using both transcription software and a human’s light touch. It has been edited for style and clarity.]
Julie Rovner: Hello, and welcome back to “What the Health?” I’m Julie Rovner, chief Washington correspondent for KFF Health News, and I’m joined by some of the best and smartest health reporters in Washington. We’re taping this week on Thursday, Jan. 25, at 10 a.m. As always, news happens fast, and things might’ve changed by the time you hear this. So here we go. We are joined today via video conference by Alice Miranda Ollstein of Politico.
Alice Miranda Ollstein: Good morning.
Rovner: Jessie Hellmann of CQ Roll Call.
Jessie Hellmann: Hi there.
Rovner: And Anna Edney of Bloomberg News.
Anna Edney: Hello.
Rovner: Later in this episode we’ll have my interview with Sarah Somers of the National Health Law Program. She’s going to explain what’s at risk for health care if the Supreme Court overturns the Chevron doctrine, and if you don’t know what that is, you will. But first, this week’s news. We’re going to start this week with politics. To absolutely no one’s surprise, Donald Trump won the first-in-the-nation New Hampshire primary, and even though he wasn’t even on the ballot, because Democrats no longer count New Hampshire as first, President [Joe] Biden handily won a write-in campaign.
Since it seems very likely at this point that the November ballot will pit Trump versus Biden once again, I thought we’d look, briefly at least, at both of their health agendas for now. Trump has once again vowed to try and repeal the Affordable Care Act, which not only didn’t go well in 2017, we learned this week that the federal marketplace enrolled a record 21.3 million people for this year. In 2017, that number was 12.2 million. Not to mention there are now a half a dozen more states that have expanded Medicaid to low-income childless adults.
So with so many more millions of Americans getting coverage via Obamacare, even if Trump wants to repeal and replace it, is there any chance Republicans would go along, even if he wins back majorities in the House and the Senate? They have seemed rather unwilling to reopen this box of worms.
Edney: I mean, certainly, I think that currently they’re unwilling. I don’t want to pretend that I know what the next several months will hold until November, but even before they’re willing or not, what would the plan be? We never saw one, and I don’t anticipate there would be any sort of real plan, particularly if it’s the Trump White House itself having to put the plan together to repeal Obamacare.
Rovner: Yes. How many times did he promise that “we’ll have a plan in two weeks” throughout most of his administration? Alice, you were saying?
Ollstein: Yes. I think what we should be thinking about, too, is this can happen not through Congress. There’s a lot of President Trump could do theoretically through the executive branch, not to repeal Obamacare, but to undermine it and make it work worse. They could slash outreach funding, they could let the enhanced tax credit subsidies expire — they’re set to expire next year. That would also be on Congress. But a president who is opposed to it could have a role in that; they could slash call center assistance. They could do a lot. So I think we should be thinking not only about could a bill get through Congress, but also what could happen at all of the federal agencies.
Rovner: And we should point out that we know that he could do some of these things because he did them in his first term.
Ollstein: He did them the first time, and they had an impact. The uninsured rate went up for the first time under Trump’s first term, for the first time since Obamacare went into effect. So it can really make a difference.
Rovner: And then it obviously went down again. But that was partly because Congress added these extra subsidies and even the Republican Congress required people to stay on Medicaid during the pandemic. Well, I know elsewhere, like on abortion, Trump has been all over the place, both since he was in office and then since he left office. And then now, Alice, do we have any idea where he is on this whole very sensitive abortion issue?
Ollstein: He has been doing something very interesting recently, which is he’s sort of running the primary message and the general message at the same time. So we’re used to politicians saying one thing to a primary audience. These are the hard-core conservatives who turn out in primaries and they want to hear abortion is going to be restricted. And then the general audience — look at how all of these states have been voting — they don’t want to hear that. They want to hear a more moderate message and so Trump has been sort of giving both at once. He’s both taking credit for appointing the Supreme Court justices, who overturned Roe v. Wade. He has said that he is pro-life, blah, blah, blah. But he has also criticized the anti-abortion movement for going too far in his view. He criticized Ron DeSantis’ six-week ban for going too far. He has said that any restrictions need to have exemptions for rape and incest, which not everyone in the movement agrees with. A lot of people disagree with that in the anti-abortion movement. And so it has been all over the place.
But his campaign is in close contact with a lot of these groups and the groups are confident that he would do what they want. So I think that you have this interesting tension right now where he is saying multiple mixed messages.
Rovner: Which he always does, and which he seems to somehow get away with. And again, just like with the ACA, we know that all of these things that he could do just from the executive branch about reproductive health, because he did them when he was president the first time. Meanwhile, President Biden, in addition to taking a victory lap on the Affordable Care Act enrollment, is doubling down on abortion and contraception, which is pretty hard because, first, as executive, he doesn’t have a ton of power to expand abortion rights the way Trump would actually have a lot of power to contract them.
And, also, because as we know, Biden is personally uncomfortable with this issue. So Alice, how well is this going to work for the Biden administration?
Ollstein: So what was announced is mostly sort of reiterating what is already the law, saying we’re going to do more to educate people about it and crack down on people who are not following it. So this falls into a few different buckets. Part of it is Obamacare’s contraception mandate. There have been lots of investigations showing that a lot of insurers are denying coverage for contraceptives they should be covering or making patients jump through hoops. And so it’s not reaching the people it should be reaching. And so they’re trying to do more on that front.
And then, on the abortion front, this is mostly in this realm of abortions in medical emergencies. They’re trying to educate patients on “you can file this complaint if you are turned away.” Of course, I’m thinking of somebody experiencing a medical emergency and needing abortion and being turned away, and I don’t think “I’m going to file an EMTALA [Emergency Medical Treatment and Active Labor Act] complaint with the federal government and hope that they do something” is maybe the first thing on their mind. But the new executive order also includes education for providers and hospitals on their obligations.
This is also something a Trump administration could completely change. They could come in and say, “Forget that guidance. Here’s our guidance, which is no abortions in these circumstances.” So this is a really sensitive issue, but I think that the Biden campaign has seen how people have been voting over the last two years and feels that this is a really good message for them to do something on.
Rovner: Meanwhile, one issue both Republicans and Democrats are trying to campaign on is bringing down the cost of prescription drugs. Stat News has a story this week suggesting that all the lawsuits against the Medicare drug negotiation program could actually help Biden with voters because it shows he’s going after Big Pharma. Frankly, it could also tell voters that the Biden administration actually did something to challenge Big Pharma. Polls show most people have no idea, but Trump can point to lots of lawsuits over things he tried to do to Big Pharma.
Does one or the other of them have an advantage here, Anna? I mean, I know they’re going in different directions, but when you sort of boil it into campaign-speak, it’s going to sound pretty similar, right?
Edney: I think that that’s true, but one of the differences is, at least currently, what Biden’s done and doing some price negotiation through Medicare so far for 10 drugs under his administration is going forward. And you can name the drugs, name the prices, talk about it a little bit more specifically. What Trump ran up against was the lawsuits not falling in his favor. So he wanted more transparency as far as the drug companies having to say the price of their drugs in TV ads, and that wasn’t able to happen. And also reference pricing, so that the prices would be benchmarked to other countries. And certainly that never went forward either. And Trump really used the going after pharma hard in the last campaign, I would say, in 2016. And it worked in the beginning, and you would see the stock of these companies start going down the second he said pharmaceutical companies are getting away with murder or whatever big comment he was making. But it eventually lost any real effect because there didn’t seem to be plans to do anything drastic.
He talked about potentially doing negotiation, like is happening currently, but then that never came to fruition once he was in office. So I don’t know if that will come across to voters, but certainly the pharma industry doesn’t seem to be as afraid of Trump as what Biden’s doing right now.
Rovner: Jessie, I know Congress is still working on this PBM [pharmacy benefit managers] transparency, big bill. Are we getting any closer to anything? I think members of Congress would also like to run on being able to say they’ve done something about prescription drug prices.
Hellmann: I was just talking to [Sen.] Chuck Grassley [R-Iowa] about this because he is the “OG PBM hater.” And he was like, “Why is nothing happening?” He was just very frustrated. There are several bills that have passed House and Senate committees, and so I think, at this point, it’s just a matter of cobbling them all together, finding ways to pay for things. And since there’s also so many other health care things that people want to get done, it’s a matter of “Do we have enough money to pay for everything? What’s going to save money? What’s going to cost money?”
There’s also these health care transparency measures that Congress is looking at. There’s this site-neutral hospital payments thing that could be a money saver. So I think there’s just a lot going on in trying to figure out how it all fits together. But PBMs, I could definitely see them doing something this year.
Rovner: Sometimes, I mean, often it’s like you can’t get things onto the agenda. In this case, it sounds like there’s lots of things on the agenda, but they’re going to need to pay for all of them and they’re going to fight over the few places where they could presumably get some savings.
Edney: I was going to say, I saw that Grassley and some other senators wrote the Federal Trade Commission because they are due for a report on PBMs they’ve been working on for about a year and a half. And I think that the senators who want to go after PBMs are kind of looking for that sort of backup and that deep dive into the industry to make those statements about cost savings and what this would do for pharmaceutical prices.
Rovner: Well, to ratchet this up one more step, the Biden administration has proposed a framework for when march-in rights might be used. Is this the real deal or a threat to get pharma to back down on complaints about the Medicare price negotiations? Anna, why don’t you explain what march-in rights are?
Edney: March-in rights, which have never been used on a pharmaceutical company, were something that were put into law — I think it was around 1980 with the Bayh-Dole Act — and what it allows the government to do is say we invested a ton of money, either through giving money to university research or in the company itself, to do the very basic science that got us to this breakthrough that then the company took across the finish line to get a drug on the market. But usually, I think the main reason you might use it is because then the company does nothing with it.
Say they bought it up and it could be a competitor to one of their drugs, so they don’t use it. But it seems like it could also be used if the price is prohibitive, that it’s something that’s really needed, but Americans aren’t getting access to it. And so the government would be able to take that patent back and lower the price on the drug. But I haven’t heard a specific drug that they want to use this on. So I don’t know if they’re serious about using the march-in rights.
There is a request for information to find out how people feel about this, how it might affect the industry. The argument being that it could hamper the innovation, but we hear that a lot from the pharmaceutical industry as well. So unclear if that’s a true defense to not using march-in rights.
Rovner: Although march-in rights are a pretty big gun. There’s a reason they’ve never been used. I’ve seen them … lawmakers sometimes trot it out kind of as a cudgel, but I’ve never … the only time I think I saw them come close was after the anthrax scare, right after 9/11, when there was potentially a shortage of the important antibiotic needed for that. There was muttering about this, but then I think the drug company decided on its own to lower the price, which got us over that.
Well, yet another tack is being pursued by Sen. Bernie Sanders, chairman of the Senate Health Committee. He’s going to make the committee vote next week on whether to subpoena the CEOs of Johnson & Johnson and Merck to require them to “provide testimony about why their companies charge substantially higher prices for medicine in the U.S. compared to other countries.” Well, we all know the answer to that. Other countries have price controls and the U.S. does not. So is this a stunt or not? And is he even going to get the rest of the committee to go along with the subpoena?
Edney: This wouldn’t be the first hearing on high drug prices pulling in CEOs. And it’s so opaque that you never get an answer. You never get something … I mean, certainly, they’ll blame PBMs and talk about that, and the finger-pointing will go somewhere else, but you never have some aha insight moment. So when the CEOs are coming in, it does feel a bit more like a show. And Bernie Sanders, the ones he wants to subpoena are from companies that are suing the Biden administration.
So there’s talk about whether that’s sort of a bit of a revenge him for that as well. I don’t know what exactly he would expect to hear from them that would change policy or what legislation they’re trying to work out by having this hearing.
Rovner: For an issue that everybody cares about, high drug prices. It sure has been hard to figure out a way into it for politicians.
Ollstein: We have seen public shaming, even without legislation behind it, can have a difference. I think we’ve seen that on the insulin front. And so I think it’s not completely a fool’s errand here, what Bernie’s trying to do. It will be interesting to see if the rest of the committee goes along with it. There’s been some tensions on the committee. There’s been bipartisan support for some of his efforts, and then others — less on the health front, I think more on the labor front — you’ve had a lot of pushback from the Republican members, and so it’ll be very telling.
Rovner: I was actually in the room when the tobacco industry CEOs came to testify at the House Energy and Commerce Committee, and that was pretty dramatic, but I feel like that was a very different kind of atmosphere than this is. I know everybody’s been trying to repeat that moment for — what is it? — 25, 30 years now. It was in the early 1990s, and I don’t think anybody really successfully has, but they’re going to keep at it.
All right, well, let us turn to abortion. Last Saturday would have been the 51st anniversary of Roe v. Wade, and the day before was the annual March for Life, the giant annual anti-abortion demonstration that used to be a march to the Supreme Court to urge the justices to overrule Roe. Well, that mission has been accomplished. So now what are their priorities, Alice?
Ollstein: Lots of things. And a lot of the effort right now is going towards laying the groundwork, making plans for a potential second Trump administration or a future conservative president. They see not that much hope on the federal level for their efforts currently, with the current president and Congress, but they are trying to do the prep work for the future. They want a future president to roll back everything Biden has done to expand abortion access. That includes the policies for veterans and military service members. That includes wider access to abortion pills through the mail and dispensing at retail pharmacies, all of that.
So they want to scrap all of that, but they also want to go a lot further and are exploring ways to use a lot of different agencies and rules and bureaucratic methods and funding mechanisms to do this, because they’re not confident in passing a bill through Congress. We’ve seen Congress not able to do that even under one-party rule in either direction. And so they’re really looking at the courts, which are a lot more conservative than they were several years ago.
Rovner: Largely thanks to Trump.
Ollstein: Exactly, exactly. So the courts, the executive branch, and then, of course, more efforts at the state level, which I know we’re going to get into.
Rovner: We are. Before that, though, one of the things that keeps coming up in discussions about the anti-abortion agenda is something called the Comstock Act. We have talked about this before, although it’s been a while, but this is an 1873 law, which is still on the books, although largely unenforced, that banned the mailing of anything that could be used to aid in an abortion, among other things. Could an anti-abortion administration really use Comstock to basically outlaw abortion nationwide?
I mean, even things that are used for surgical abortion tend to come through … it’s not just the mail, it’s the mail or FedEx or UPS, common carrier.
Ollstein: Yes. So this is getting a lot more attention now and it is something anti-abortion groups are absolutely calling for, and people should know that this wouldn’t only prohibit the mailing of abortion pills to individual patients’ homes, which is increasingly happening now. This would prevent it from being mailed to clinics and medical facilities. The mail is the mail. And so because abortion medication is used in more than half of all abortions nationwide, it could be a fairly sweeping ban.
And so the Biden administration put out a memo from the Justice Department saying, “Our interpretation of the Comstock Act is that it does not prohibit the mailing of abortion pills.” The Trump administration or whoever could come in and say, “We disagree. Our interpretation is that it does.” Now, how they would actually enforce it is a big question. Are you going to search everyone’s mail in the country? Are you going to choose a couple of people and make an example out of them?
That’s what happened under the original Comstock Act. Back in the day, they went after a few high-profile abortion rights activists and made an example out of them. I think nailing them down on how it would be enforced is key here. And of course there would be tons of legal challenges and battles no matter what.
Rovner: Absolutely. Well, let us turn to the states. It’s January, which is kind of “unveil your bills” time in state legislatures, and they are piling up. In Tennessee, there’s a bill that would create a Class C felony, calling for up to 15 years in prison, for an adult who “recruits, harbors or transports a pregnant minor out of state for an abortion.” There’s a similar bill in Oklahoma, although violators there would only be subject to five years in prison.
Meanwhile, in Iowa, Republican lawmakers who are writing guidelines for how to implement that state’s six-week ban, which is not currently in effect, pending a court ruling, said that the rape exception could only be used if the rape is “prosecutable,” without defining that word. Are these state lawmakers just failing to read the room or do they think they are representing what their voters want?
Edney: I don’t really know. I think clearly there are a lot of right-wing Republicans who are elected to office and feel that they have a higher calling that doesn’t necessarily reflect what their constituents may or may not want, but more is that they know better. And I think that that could be some of this, because certainly the anti-abortion bills or movements have been rejected by voters in places you might not exactly expect it.
Rovner: It feels like we’re getting more and more really “out there” ideas on the anti-abortion side at the same time that we’re getting more and more ballot measures of voters in both parties wanting to protect abortion rights, at least to some extent.
Ollstein: And I think going off what Anna said, I think that anti-abortion leaders, including lawmakers, are being more upfront now, saying that they don’t believe that this should be something that the democratic process has a voice in. The framing they use is that fetuses are an oppressed minority and their rights should not be subject to a majority vote. That’s their framing, and they’re being very upfront saying that these kinds of ballot referendums shouldn’t be allowed, and that states that do allow them should get rid of that. We’ll see if that happens. There are obviously lots of attempts to thwart specific state efforts to put abortion on the ballot. There are lawsuits pending in Nevada and Florida. There are attempts to raise the signature threshold, raise the vote threshold, just make it harder to do overall. But I found it very interesting and a pretty recent development that folks are coming out and saying the quiet part out loud. Saying, “We don’t believe The People should be able to decide this.”
Rovner: Well, obviously not an issue that is going away anytime soon. All right, well that is this week’s news. Now we will play my interview with Sarah Somers, and then we will come back and do our extra credits.
I am pleased to welcome to the podcast Sarah Somers, legal director of the National Health Law Program. She’s going to explain, in English hopefully, what’s at stake in the big case the Supreme Court heard earlier this month about herring fishing. Sarah, welcome to “What the Health?”
Sarah Somers: Thank you for having me, Julie. I’m glad to be here.
Rovner: So this case, and I know it’s actually two cases together, is really about much more than herring fishing, right? It seems to be about government regulation writ large.
Somers: That’s right. The particular issue in the case is about a national marine fisheries regulation that requires herring fishing companies to pay for observers who are on board — not exactly an issue that’s keeping everyone but herring fishermen up at night. And the fishing company challenged the rule, saying that it wasn’t a reasonable interpretation of the statute. But what they also asked the court to do was to overrule a Supreme Court case that requires courts to defer to reasonable agency interpretations of federal statutes. That’s what’s known as “Chevron deference.”
Rovner: And what is Chevron deference and why is it named after an oil company?
Somers: Why aren’t we talking about oil now? Yes, Chevron deference is the rule that says that courts have to defer to a reasonable agency interpretation of a federal statute. So, under Chevron, there’s supposed to be a two-step process when considering whether, say, a regulation is a reasonable interpretation. They say, “Does the statute speak directly to it?” So in this case, did the statute talk about whether you have to pay for observers on herring boats? It didn’t.
So the next question was, if it doesn’t speak directly to it or if it’s ambiguous or unclear, then the court should defer to a reasonable interpretation of that statute. And what’s reasonable depends on what the court determines are sort of the bounds of the statute, whether the agency had evidence before it that supported it, whether it showed the proper deliberation and expertise.
Rovner: One of the reasons that regulations are sometimes 200 pages long, right?
Somers: Exactly. And sometimes courts do say, “You know what? The statute spoke right to this. We don’t have to go any further. We know what Congress wanted.” Other times they take a step further. And the reason it’s called Chevron is it’s named after a case that was decided 40 years ago in 1984 during the Reagan administration, and it was Chevron Inc. USA v. the Natural Resources Defense Council. That case was about a regulation interpreting the Clean Air Act and about regulating air pollution.
Rovner: So, as you point out, you helped write one of the amicus briefs in the case about what overturning Chevron would mean for health care. It’s not just about herring fishing and Clean Air Act. Can you give us the CliffsNotes version of what it would mean for health care?
Somers: One of the purposes of our amicus brief was just to give another angle on this, because we were talking a lot about regulations in the context of air pollution, clean water, and the environment, but it touches so many other things, and this is just one aspect of it. So this brief, which we authored along with the American Cancer Society Action Network, and a Boston law firm called Anderson Kreiger, was signed by other health-oriented groups: the American Lung Association, American Heart Association, Campaign for Tobacco-Free [Kids], and then the American Academy of Pediatrics, American Academy of Public Health.
You get the picture. These are all groups that have a vested interest in programs of the Department and Health and Human Services. The brief talks about regulations promulgated by the Centers for Medicare & Medicaid Services. I’m going to call them CMS for short when we’re talking. And CMS is responsible for regulating the vast and complex Medicare and Medicaid programs. And, as you know, Medicare and Medicaid cover more than half of the population and touch the lives of almost everyone, regulating hospitals, some aspects of insurance, some aspects of practice of medicine.
You can’t escape the consequences of problems with these programs. And so that’s why the agency … Congress specifically gave HHS and CMS the power to regulate all of the issues in its purview. So that already have the power, and so the question is whether they use it wisely. We are arguing in this brief that for 40 years it’s worked just fine. That Congress has set the outer limits and been content to let the agency determine the specifics of these programs to fill in the gaps, as one Supreme Court case said. And this has implications for how hospitals operate, how insurance programs operate, and whether they operate smoothly.
And in our brief, we’re not really arguing for or against a particular interpretation or either for or against what the agency says. It’s just a matter of stability and certainty. The agency has the expertise, has the time, has the resources, and has the duty to figure out what these particular terms and statutes mean and how the programs should work. Just two examples we gave in the brief of the kind of issues that the agency should be determining are: What’s the definition of geographic area in the Medicaid Act for the purpose of setting hospital wages?
If your listeners are still listening, I hope, because that is boring, arcane, hyper-technical, and courts don’t have the expertise, much less the time, to do that. And CMS does. Or another question in a different area, whether feeding activities in a nursing home regulated by Medicaid: Are those nursing or nursing related services? The court’s not going to know. The courts doesn’t have expertise or time. And again, that’s what CMS is for.
So not only is this something that you need these interpretations in these rules to have the programs operate smoothly and consistently, and that’s the first part that’s important. But the second part is that you need consistency across the country. As you know well, there are hospital systems that operate across multi-states. There are Medicaid managed-care plans operating across multi-states. All aspects of health care is nationalized. If you have hundreds of district courts and courts of appeals coming up with different interpretations of these terms, you’re going to have a lot of problems. It’s not going to operate smoothly. So I heard some of the justices arguing, “Well, Congress just needs to do its job.”
Congress has obstacles to doing even the big, mega issues that are before them, these kinds of arcane specific issues. They don’t have the time or again, the expertise. That’s why they said, “CMS, you go do this.”
Rovner: When they were writing the Affordable Care Act, there were so many times in that legislation where it says, “The secretary shall” or “The secretary may.” It’s like, we’re going to punt all this technical stuff to HHS and let them do what they will.
Somers: Exactly. You figure out what the definition of a preventive service is, that’s not something that we are going to do. And there are also questions raised about is this … these unelected agency personnel, well, agencies — they are political appointees, and they also serve at the pleasure of the head of the agency. So they’re accountable to the executive branch and indirectly to the voters. The courts, at this point, once they’re on the court and the federal courts, they’re not accountable to the voters anymore. And so this would be a big shift of power towards the courts, and that is what we argued would be antithetical to the system working well.
Rovner: What would be an example of something that could get hung up in the absence of Chevron?
Somers: I thought that Justice [Ketanji Brown] Jackson, during the argument, gave a really good example. Under the Food and Drug Administration’s power to regulate new drugs and determining what is an adequate and well-controlled investigation. The idea of courts, every single drug that’s challenged in every single forum, having to delve into what that means without deference to the agency would be just a recipe for chaos, really.
Rovner: So some people have argued that Chevron is already basically gone, as far as the Supreme Court is concerned, that it’s been replaced by the major questions doctrine, which is kind of what it sounds like. If a judge thinks a question is major, and they will assume that the Congress has not delegated it to the agency to interpret. So what difference would it make if the court formally overturned Chevron or not here? I guess what you’re getting at is that we’re more worried about the lower courts at this point than the Supreme Court, right?
Somers: That’s right. The Supreme Court has not cited Chevron in something like 15 years. And they talked about that in the argument, but it’s for the lower courts. The lower courts still follow it. It is still very commonly cited and gives them a lot of guidance not to have to decide these issues in the first instance. It’s true that the major questions doctrine — and there are other threats to the power of the administrative agencies, and we should all be concerned about them. But this one is really the grease that keeps the machine going and keeps these systems going. And throwing all that up in the air would make a big difference. If only because the question in all of these Chevron cases, and so many of them was not the ultimate issue — about whether the regulation was a good policy — but the question was, was the statute ambiguous or not? And so that’s the part that would be up in the air and everyone can go back and re-litigate these, including the big interests that have a lot of time and resources to devote to litigation. And that would cause a great deal of uncertainty, a lot of disruption, and a lot of problem for the courts and for all the entities that function under these systems.
Rovner: And that’s a really important point. It’s not just going forward. People who are unhappy with what a regulation said could go back, right?
Somers: Oh yeah. They could go back. They could go to different courts. We’ve seen how litigants can forum-shop. They can find a judge that they think is going to be sympathetic to their argument and make a determination that affects the whole country.
Rovner: Well, we will be watching. Sarah Somers, thanks so much for joining us.
Somers: My pleasure. Thank you for having me.
Rovner: We are back, and it’s time for our extra-credit segment. That’s when we each recommend a story we read this week we think you should read, too. As always, don’t worry if you miss it. We will post the links on the podcast page at kffhealthnews.org and in our show notes on your phone or other mobile device. Jessie, you were the first to join in this week. Why don’t you tell us about your extra credit?
Hellmann: Yeah. Mine is from North Carolina Health News. They wrote about how congenital syphilis is killing babies in the state. They had eight cases of deaths last year — compared to a decade ago, they had one. So it’s something that’s been on the rise in North Carolina, but also nationwide, and it’s caused a lot of alarm among public health officials because it’s pretty preventable. It’s something that doesn’t need to happen, but the story is about what the state is doing to improve their outreach to pregnant people. They’re doing media campaigns, they’re trying to make sure that people are doing their prenatal care and just trying to stop this from happening. So I thought that was a good story. It’s definitely kind of an under-reported issue. It’s something that public health officials have been raising an alarm about for a while now, but there’s just not enough funding or attention on the issue.
Rovner: For all the arguing about abortion, there’s not been a lot of discussion about maternal and child health, which obviously appears to be the one place that both sides agree on. Anna.
Edney: Mine’s in The New Yorker by Rachael Bedard. It’s “What Would It Mean for Scientists to Listen to Patients?” And it’s interesting, it’s about two Yale researchers who are doing a long-covid study, but it’s unique in the sense that when the CDC or anyone else does a long-covid study, they typically are trying to say, “Here are the exact symptoms. We’re going to work with 12 of them.” Whereas we know long covid, it’s seemingly a much more expansive symptom list than that, but researchers really like to have kind of metrics to go by.
But what these Yale researchers are doing is letting all of that go and just letting anybody in this and talking to them. They’re holding monthly town halls with people who are in this, whoever wants to show up and come and just talk to them about what’s going on with them and trying to find out, obviously, what could help them. But they’re not giving medical advice during these, but just listening. And it just was so novel, and maybe it shouldn’t be, but I found it fascinating to read about and to get their reactions. And it’s not always easy for them. I mean, the patients get upset and want something to happen faster, but just that somebody is out there doing this research and including anybody who feels like they have long covid. It was really well-written too.
Rovner: It’s a really good story. Alice.
Ollstein: So I’m breaking my streak of extremely depressing, grim stories and sharing kind of a funny one, although it could have some serious implications. This is from Stat, and it’s from an inspector general report about how the White House pharmacy, which is run by basically the military, functioned under President Trump. And it functioned like sort of a frat house. There was no official medical personnel in charge of handing out the medications, and they were sort of handed out to whoever wanted them, including people who shouldn’t have been getting them. People were just rifling through bins of medications and taking what they wanted. These included pills like Ambien and Provigil, sort of uppers and downers in the common parlance. And so I think this kind of scrutiny on something that I didn’t even know existed. The White House pharmacy is pretty fascinating.
Rovner: It was a really, really interesting story. Well, I also have something relatively hopeful. My extra credit this week is a journal article from Health Affairs with the not-so-catchy headline “‘Housing First’ Increased Psychiatric Care Office Visits and Prescriptions While Reducing Emergency Visits,” by Devlin Hanson and Sarah Gillespie. And if they will forgive me, I would rename it, calling it maybe “Prioritizing Permanent Housing for Homeless People Provides Them a Better Quality of Life at Potentially Less Cost to the Public.”
It’s about a “Housing First” experiment in Denver, which found that the group that was given supportive housing was more likely to receive outpatient care and medications and less likely to end up in the emergency room. The results weren’t perfect. There was no difference in mortality between the groups that got supportive housing and the groups that didn’t. But it does add to the body of evidence about the use of so-called social determinants of health, and how medicine alone isn’t the answer to a lot of our social and public health ills.
OK. That is our show. As always, if you enjoy the podcast, you can subscribe wherever you get your podcasts. We’d appreciate it if you left us a review; that helps other people find us, too. Special thanks as always to our technical guru, Francis Ying, and our editor, Emmarie Huetteman. As always, you can email us your comments or questions. We’re at whatthehealth@kff.org, or you can still find me at X, @jrovner, or @julierovner at Bluesky or @julie.rovner at Threads. Anna, where are you these days?
Edney: Mostly just on Threads, so @anna_edneyreports.
Rovner: Alice?
Ollstein: @AliceOllstein.
Rovner: Jessie.
Hellmann: @jessiehellmann on Twitter.
Rovner: We will be back in your feed next week. Until then, be healthy.
Credits
Francis Ying
Audio producer
Emmarie Huetteman
Editor
To hear all our podcasts, click here.
And subscribe to KFF Health News’ “What the Health?” on Spotify, Apple Podcasts, Pocket Casts, or wherever you listen to podcasts.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 6 months ago
Courts, Elections, Health Care Costs, Multimedia, Pharmaceuticals, Public Health, States, The Health Law, Abortion, Drug Costs, KFF Health News' 'What The Health?', New Hampshire, Podcasts, Women's Health
What the Health Care Sector Was Selling at the J.P. Morgan Confab
SAN FRANCISCO — Every year, thousands of bankers, venture capitalists, private equity investors, and other moneybags flock to San Francisco’s Union Square to pursue deals. Scores of security guards keep the homeless, the snoops, and the patent-stealers at bay, while the dealmakers pack into the cramped Westin St.
Francis hotel and its surrounds to meet with cash-hungry executives from biotech and other health care companies. After a few years of pandemic slack, the 2024 J.P. Morgan Healthcare Conference regained its full vigor, drawing 8,304 attendees in early January to talk science, medicine, and, especially, money.
1. Artificial Intelligence: Revolutionary or Not?
Of the 624 companies that pitched at the four-day conference, the biggest overflow crowd may have belonged to Nvidia, which unlike the others isn’t a health care company. Nvidia makes the silicon chips whose computing power, when paired with ginormous catalogs of genes, proteins, chemical sequences, and other data, will “revolutionize” drug-making, according to Kimberly Powell, the company’s vice president of health care. Soon, she said, computers will customize drugs as “health care becomes a technology industry.” One might think that such advances could save money, but Powell’s emphasis was on their potential for wealth creation. “The world’s first trillion-dollar drug company is out there somewhere,” she dreamily opined.
Some health care systems are also hyping AI. The Mayo Clinic, for example, highlighted AI’s capacity to improve the accuracy of patient diagnoses. The nonprofit hospital system presented an electrocardiogram algorithm that can predict atrial fibrillation three months before an official diagnosis; another Mayo AI model can detect pancreatic cancer on scans earlier than a provider could, said Matthew Callstrom, chair of radiology at the Mayo Clinic in Rochester, Minnesota.
No one really knows how far — or where — AI will take health care, but Nvidia’s recently announced $100 million deal with Amgen, which has access to 500 million human genomes, made some conference attendees uneasy. If Big Pharma can discover its own drugs, “biotech will disappear,” said Sherif Hanala of Seqens, a contract drug manufacturing company, during a lunch-table chat with KFF Health News and others. Others shrugged off that notion. The first AI algorithms beat clinicians at analyzing radiological scans in 2014. But since that year, “I haven’t seen a single AI company partner with pharma and complete a phase I human clinical trial,” said Alex Zhavoronkov, founder and CEO of Insilico Medicine — one of the companies using AI to do drug development. “Biology is hard.”
2. Weight Loss Pill Profits and Doubts
With predictions of a $100 billion annual market for GLP-1 agonists, the new class of weight loss drugs, many investors were asking their favorite biotech entrepreneurs whether they had a new Ozempic or Mounjaro in the wings this year, Zhavoronkov noted. In response, he opened his parlays with investors by saying, “I have a very cool product that helps you lose weight and gain muscle.” Then he would hand the person a pair of Insilico Medicine-embossed bicycle racing gloves.
More conventional discussions about the GLP-1s focused on how insurance will cover the current $13,000 annual cost for the estimated 40% of Americans who are obese and might want to go on the drugs. Sarah Emond, president of the Institute for Clinical and Economic Review, which calculates the cost and effectiveness of medical treatments, said that in the United Kingdom the National Health Service began paying in 2022 for obese patients to receive two years of semaglutide — something neither Medicare nor many insurers are covering in the U.S. even now.
But studies show people who go off the drugs typically regain two-thirds of what they lose, said Diana Thiara, medical director for the University of California-San Francisco weight management program. Recent research shows that the use of these drugs for three years reduces the risk of death, heart attack, and stroke in non-diabetic overweight patients. To do right by them, the U.S. health care system will have to reckon with the need for long-term use, she said. “I’ve never heard an insurer say, ‘After two years of treating this diabetes, I hope you’re finished,’” she said. “Is there a bias against those with obesity?”
3. Spotlight on Tax-Exempt Hospitals
Nonprofit hospitals showed off their investment appeal at the conference. Fifteen health systems representing major players across the country touted their value and the audience was intrigued: When headliners like the Mayo Clinic and the Cleveland Clinic took the stage, chairs were filled, and late arrivals crowded in the back of the room.
These hospitals, which are supposed to provide community benefits in exchange for not paying taxes, were eager to demonstrate financial stability and showcase money-making mechanisms besides patient care — they call it “revenue diversification.” PowerPoints skimmed through recent operating losses and lingered on the hospital systems’ vast cash reserves, expansion plans, and for-profit partnerships to commercialize research discoveries.
At Mass General Brigham, such research has led to the development of 36 drugs currently in clinical trials, according to the hospital’s presentation. The Boston-based health system, which has $4 billion in committed research funding, said its findings have led to the formation of more than 300 companies in the past decade.
Hospital executives thanked existing bondholders and welcomed new investors.
“For those of you who hold our debt, taxable and tax-exempt, thank you,” John Mordach, chief financial officer of Jefferson Health, a health system in Pennsylvania and New Jersey. “For those who don’t, I think we’re a great, undervalued investment, and we get a great return.”
Other nonprofit hospitals talked up institutes to draw new patients and expand into lucrative territories. Sutter Health, based in California, said it plans to add 30 facilities in attractive markets across Northern California in the next three years. It expanded to the Central Coast in October after acquiring the Sansum Clinic.
4. Money From New — And Old — Treatments for Autoimmune Disease
Autoimmunity drugs, which earn the industry $200 billion globally each year, were another hot theme, with various companies talking up development programs aimed at using current cancer drug platforms to create remedies for conditions like lupus and rheumatoid arthritis. AbbVie, which has led the sector with its $200 billion Humira, the world’s best-selling drug, had pride of place at the conference with a presentation in the hotel’s 10,000-square-foot Grand Ballroom.
President Robert Michael crowed about the company’s newer autoimmune drugs, Skyrizi and Rinvoq, and bragged that sales of two-decades-old Humira were going “better than anticipated.” Although nine biosimilar — essentially, generic — versions of the drug, adalimumab, entered the market last year, AbbVie expects to earn more than $7 billion on Humira this year since the “vast majority” of patients will remain on the market leader.
In its own presentation, biosimilar-maker Coherus BioSciences conceded that sales of Yusimry, its Humira knockoff listed at one-seventh the price of the original, would be flat until 2025, when Medicare changes take effect that could push health plans toward using cheaper drugs.
Biosimilars could save the U.S. health care system $100 billion a year, said Stefan Glombitza, CEO of Munich-based Formycon, another biosimilar-maker, but there are challenges since each biosimilar costs $150 million to $250 million to develop. Seeing nine companies enter the market to challenge Humira “was shocking,” he said. “I don’t think this will happen again.”
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 6 months ago
california, Health Industry, Pharmaceuticals, States, Health IT, Hospitals, Prescription Drugs
STAT+: Klobuchar urges drugmakers to remove patents FTC calls improper and inaccurate
Amid a push to crack down on patent abuse by the pharmaceutical industry, a key U.S. lawmaker is urging six large drug companies to remove dozens of patents that were identified by regulators as improperly or inaccurately listed with a federal registry.
In a series of letters sent on Thursday, Sen. Amy Klobuchar (D-Minn.) demanded the companies explain why they have, so far, not responded to warnings issued two months ago by the Federal Trade Commission to remove more than 100 patents from the registry. The agency threatened the drug companies with litigation if they failed to comply.
The FTC had challenged a total of 10 companies over listings for patents on such medicines as asthma inhalers and epinephrine auto-injectors as part of an effort to mitigate actions that thwart competition. Among the companies to which the agency sent warnings are AbbVie, AstraZeneca, Mylan Specialty, Boehringer Ingelheim, and subsidiaries of GSK and Teva Pharmaceutical.
1 year 6 months ago
Pharma, Pharmalot, patents, Pharmaceuticals, STAT+
KFF Health News' 'What the Health?': 2023 Is a Wrap
The Host
Julie Rovner
KFF Health News
Julie Rovner is chief Washington correspondent and host of KFF Health News’ weekly health policy news podcast, “What the Health?” A noted expert on health policy issues, Julie is the author of the critically praised reference book “Health Care Politics and Policy A to Z,” now in its third edition.
Even without covid dominating the headlines, 2023 was a busy year for health policy. The ever-rising cost of health care remained an issue plaguing patients and policymakers alike, while millions of Americans lost insurance coverage as states redetermined eligibility for their Medicaid programs in the wake of the public health emergency.
Meanwhile, women experiencing pregnancy complications continue to get caught up in the ongoing abortion debate, with both women and their doctors potentially facing prison time in some cases.
This week’s panelists are Julie Rovner of KFF Health News, Rachel Cohrs of Stat, Sandhya Raman of CQ Roll Call, and Joanne Kenen of Johns Hopkins University and Politico Magazine.
Panelists
Rachel Cohrs
Stat News
Joanne Kenen
Johns Hopkins Bloomberg School of Public Health and Politico
Sandhya Raman
CQ Roll Call
Among the takeaways from this week’s episode:
- As the next election year fast approaches, the Biden administration is touting how much it has accomplished in health care. Whether the voting public is paying attention is a different story. Affordable Care Act enrollment has reached record levels due in part to expanded financial help available to pay premiums, and the administration is also pointing to its enforcement efforts to rein in high drug prices.
- The federal government is adding staff to go after “corporate greed” in health care, targeting in particular the fast-growing role of private equity. The complicated, opaque, and evolving nature of corporate ownership in the nation’s health system makes legislation and regulation a challenge. But increased interest and oversight could lead to a better understanding of the problems of and, eventually, remedies for a profit-focused system of health care.
- Concluding a year that saw many low-income Americans lose insurance coverage as states reviewed eligibility for everyone in the Medicaid program, there’s no shortage of access issues left to tackle. The Biden administration is urging states to take action to help millions of children regain coverage that was stripped from them.
- Also, many patients are all too familiar with the challenges of obtaining insurance approval for care. There is support in Congress to scrutinize and rein in the use of algorithms to deny care to Medicare Advantage patients based on broad comparisons rather than individual patient circumstances.
- And in abortion news, some conservative states are trying to block efforts to put abortion on the ballot next year — a tactic some used in the past against Medicaid expansion.
- This week in health misinformation is an ad from Florida’s All Family Pharmacy touting the benefits of ivermectin for treating covid-19. (Rigorous scientific studies have found that the antibacterial drug does not work against covid and should not be used for that purpose.)
Also this week, Rovner interviews KFF Health News’ Jordan Rau about his joint KFF Health News-New York Times series “Dying Broke.”
Plus, for “extra credit,” the panelists suggest health policy stories they read this week that they think you should read, too:
Julie Rovner: Business Insider’s “‘I Feel Conned Into Keeping This Baby,’” by Bethany Dawson, Louise Ridley, and Sarah Posner.
Joanne Kenen: The Trace’s “Chicago Shooting Survivors, in Their Own Words,” by Justin Agrelo.
Rachel Cohrs: ProPublica’s “Doctors With Histories of Big Malpractice Settlements Work for Insurers, Deciding if They’ll Pay for Care,” by Patrick Rucker, The Capitol Forum; and David Armstrong and Doris Burke, ProPublica.
Sandhya Raman: Roll Call’s “Mississippi Community Workers Battle Maternal Mortality Crisis,” by Lauren Clason.
Also mentioned in this week’s episode:
- Stat News’ “Humana Used Algorithm in ‘Fraudulent Scheme’ to Deny Care to Medicare Advantage Patients, Lawsuit Alleges,” by Casey Ross and Bob Herman.
- USA Today’s “Cigna Denied a Lung Transplant for a Cancer Patient. Insurer Now Says That Was an Eerror.’ By Ken Alltucker.
- Politico’s “Conservatives Move to Keep Abortion off the 2024 Ballot,” by Alice Miranda Ollstein and Megan Messerly.
- The New York Times’ “Why Democracy Hasn’t Settled the Abortion Question,” by Kate Zernike.
click to open the transcript
Transcript: 2023 Is a Wrap
KFF Health News’ ‘What the Health?’Episode Title: 2023 Is a WrapEpisode Number: 327Published: Dec. 21, 2023
[Editor’s note: This transcript was generated using both transcription software and a human’s light touch. It has been edited for style and clarity.]
Julie Rovner: Hello, and welcome back to “What the Health?” I’m Julie Rovner, chief Washington correspondent for KFF Health News, and I’m joined by some of the best and smartest health reporters in Washington. We’re taping this week on Thursday, Dec. 21, at 10 a.m. As always, news happens fast and things might’ve changed by the time you hear this, so here we go.
We are joined today via video conference by Joanne Kenen of the Johns Hopkins University and Politico Magazine.
Joanne Kenen: Hi, everybody.
Rovner: Sandhya Raman of CQ Roll Call.
Sandhya Raman: Good morning.
Rovner: And Rachel Cohrs of Stat News.
Rachel Cohrs: Hi.
Rovner: Later in this episode, we’ll have my interview with KFF Health News’ Jordan Rau, co-author of a super scary series done with The New York Times about long-term care. It’s called “Dying Broke.” But first, this week’s news. I thought we would try something a little bit different this week. It just happened that most of this week’s news also illustrates themes that we’ve been following throughout the year. So we get a this-week update plus a little review of the last 12 months, since this is our last podcast of the year. I want to start with the theme of, “The Biden administration has gotten a ton of things done in health, but nobody seems to have noticed.”
We learned this week that, with a month still to go, Affordable Care Act plan sign-ups are already at historic highs, topping 15 million, thanks, at least in part, to extra premium subsidies that the administration helped get past this Congress and which Congress may or may not extend next year. The administration has also managed to score some wins in the battle against high drug prices, which is something that has eluded even previous Democratic administrations. Its latest effort is the unveiling of 48 prescription drugs officially on the naughty list — that’s my phrase, not theirs — for having raised their prices by more than inflation during the last quarter of this year, and whose manufacturers may now have to pay rebates. This is something in addition to the negotiations for the high-priced drugs, right, Rachel?
Cohrs: Yeah, this was just a routine announcement about the drugs that are expected to be charged rebates and drugmakers don’t have to pay immediately; I think they’re kind of pushing that a little further down the road, as to when they’ll actually invoice those rebates. But the announcement raised a question in my mind of — certainly they want to tout that they’re enforcing the law; that’s been a big theme of this year — but it brought up a question for me as to whether the law is working to deter price hikes if these companies are all doing it anyway, so just a thought.
Rovner: It is the first year.
Cohrs: It is. This started going into effect at the end of last year, so it’s been a little over a year, but this is assessed quarterly, so the list has grown as time has gone on. But just a thought. Certainly there’s time for things to play out differently, but that’s at least what we’ve seen so far.
Rovner: They could say, which they did this week, it’s like, Look, these are drugs because they raised the prices, they’re going to have to give back some of that money. At least in theory, they’re going to have to give back some of that money.
Cohrs: In Medicare.
Rovner: Right. In Medicare. Some of this is still in court though, right?
Cohrs: Yes. So I think at any moment, I think this has been a theme of this year and will be carrying into next year, that there are several lawsuits filed by drugmakers, by trade associations, that just have not been resolved yet, and I think some of the cases are close to being fully briefed. So we may see kind of initial court rulings as to whether the law as a whole is constitutional. It is worth noting that most of those lawsuits are solely challenging the negotiation piece of the law and not the inflation rebates, but this could fall apart at any moment. There could be a stay, and I expect that the first court ruling is not going to be the last. There’s going to be a long appeals process. Who knows how long it’s going to take, how high it will go, but I think there is just a lot of uncertainty around the law as a whole.
Rovner: So the administration gets to stand there and say, “We did something about drug prices,” and the drug companies get to stand there and say, “Not yet you didn’t.”
Cohrs: Exactly. Yes, and they can both be correct.
Rovner: That’s basically where we are.
Cohrs: Yes.
Rovner: That’s right. Well, meanwhile, in other news from this week and from this year, the Federal Trade Commission, the Department of Justice, and the Department of Health and Human Services are all adding staff to go after what Biden officials call “corporate greed” — that is their words — in health care. Apparently these new staffers are going to focus on private equity ownership of health care providers, something we have talked about a lot and so-called roll-ups, which we haven’t talked about as much. Somebody explain what a roll-up is please.
Kenen: Julie, why don’t you?
Rovner: OK. I guess I’m going to explain what a roll-up is. I finally learned what a roll-up is. When companies merge and they make a really big company, then the Federal Trade Commission gets to say, “Mmm, you may be too big, and that’s going to hurt trade.” What a roll-up is is when a big company goes and buys a bunch of little companies, so each one doesn’t make it too big, but together they become this enormous — either a hospital system or a nursing home system or something that, again, is not necessarily going to make free trade and price limits by trade happen. So this is something that we have been seeing all year. Can the government really do anything about this? This also feels like sort of a lot of, in theory, they can do these things and in practice it’s really hard.
Cohrs: I feel like what we’ve seen in this space — I think my colleague Brittany wrote about kind of this move — is that the corporate structures around these entities are so complicated. Is it going to discourage companies from doing anything by hiring a couple people? Probably not. But I think the people power behind understanding how these structures work can lay the groundwork for future steps on understanding the landscape, understanding the tactics, and what we see, at least on the congressional side, is that a lot of times Congress is working 10 years behind some of the tactics that these companies are using to build market power and influence prices. So I think the more people power, the better, in terms of understanding what the most current tactics are, but it doesn’t seem like this will have significant immediate difference on these practices.
Kenen: I think that the gap between where the government is and where the industry is is so enormous. I think the role of PE [private equity] in health care has grown so fast in a relatively short period of time. Was there a presence before? Yes, but it’s just really taken off. So I think that if those who advocate for greater oversight, if they could just get some transparency, that would be their win, at the moment. They cannot go in and stop private equity. They would like to get to the point where they could curb abuse or set parameters or however you want to phrase it, and different people would phrase it in different ways, but right now they don’t even really know what’s going on. So, even among the Democrats, there was a fight this year about whether to include transparency language between [the House committees on] Energy and Commerce and Ways and Means, and I don’t think that was ever resolved.
I think that’s part of the “Let’s do it in January” mess. But I think they just sort of want not only greater insight for the government, but also for the public: What is going on here and what are its implications? People who criticize private equity — the defenders can always find some examples of companies that are doing good things. They exist. We all know who the two or three companies we hear all the time are, but I think it’s a really enormous black box, and not only is it a black box, but it’s a black box that’s both growing and shifting, and getting into areas that we didn’t anticipate a few years ago, like ophthalmology. We’ve seen some of these studies this year about specialties that we didn’t think of as PE targets. So it’s a big catch-up for roll-up.
Rovner: Yeah, and I think it’s also another place that the administration — and I think the Trump administration tried to do this too. Republicans don’t love some of these things either. The public complains about high health care costs. They’re right; we have ridiculously high health care costs in this country, much higher than in other countries, and this is one of the reasons why, is that there are companies going in who are looking to simply do it to make a profit and they can go in and buy these things up and raise prices. That’s a lot of what we’re seeing and a lot of why people are so frustrated. I think at very least it at least shows them: It’s like, “See, this is what’s happening, and this is one of the reasons why you’re paying so much.”
Kenen: It’s also changing how providers and practitioners work, and how much autonomy they have and who they work for. It’s in an era when we have workforce shortages in some sectors and burnout and dissatisfaction. There are pockets at least, and again, we don’t really know how big, because we don’t have our arms around this, but there are pockets; at least we do know where the PE ownership and how they dictate practice is worsening these issues of burnout and dissatisfaction. I’m having dinner tomorrow night with a expert on health care antitrust, so if we were doing this next week, I would be so much smarter.
Rovner: We will be sure to call on you in January. Workforce burnout: This is another theme that we’ve talked about a lot this year.
Kenen: It’s getting into places you just wouldn’t think. I was talking to a physical therapist the other day and her firm has been bought up, and it’s changing the way she practices and her ability to make decisions and how often she’s allowed to see a patient.
Rovner: Yeah. Well, another continuing theme. Well, yet another big issue this year has been the so-called Medicaid unwinding, as states redetermine eligibility for the first time since the pandemic began. All year, we’ve been hearing stories about people who are still eligible being dropped from the rolls, either mistakenly or because they failed to file paperwork they may never have received. Among the more common mistakes that states are making is cutting off children’s coverage because their parents are no longer eligible, even though children are eligible for coverage up to much higher family incomes than their parents. So even if the parents aren’t eligible anymore, the children most likely are.
This week, the federal government reached out to the nine states that have the highest rates of discontinuing children’s coverage, including some pretty big states, like Texas and Florida, urging them to use shortcuts that could get those children’s insurance back. But this has been a push-and-pull effort all year between the states and the federal government, with the feds trying not to push too hard. At one point, they wouldn’t even tell us which states they were sort of chiding for taking too many people off too fast. And it feels like some of the states don’t really want to have all these people on Medicaid and they would just as soon drop them even if they might be eligible. Is that kind of where we are?
Raman: You can kind of look to see the tea leaves at what some of these states are. The states that the health secretary wrote to, that have 60% of the decline in the kids being disenrolled, align pretty well with the states that have not expanded Medicaid. So they’re already going to have much fewer people enrolled than states where the eligibility levels are a lot more generous. So it’s not surprising, and some of these states have been just a little bit more aggressive from the get-go or said that they wanted to do the eligibility redeterminations a lot faster than some of the other states that wanted to take the longer time, reevaluate different ways to see if someone was still eligible, whether they were maybe getting SNAP [Supplemental Nutrition Assistance Program] benefits or other things like that. So it’s not surprising.
Rovner: You mean do it more carefully.
Raman: Yeah, yeah, so I think that the letter is one step, but if those states are really going to take up implementing these other strategies to kind of decrease that drop-off, unclear, just because they have been pretty proactive about doing this in a quick process.
Rovner: I also noticed that the states that the HHS secretary wrote to kind of tracked with the states that didn’t expand Medicaid under the Affordable Care Act, but interestingly, that meant that there would’ve been fewer parents who were eligible in the first place. So there shouldn’t have been as many children cut off, because there weren’t as many parents who ever got onto Medicaid in those states, which is why it made me raise my eyebrows a little bit. Again, I think this is something that we shall continue to follow going into next year.
Kenen: But we should also point out that even the more pro-Medicaid, liberal states have not done a great job with unwinding. It’s been bumpy pretty much across the board. It’s been very problematic. It’s a clumsy process in a normal year, and trying to catch up on three years’ worth — this is a population where people’s income varies a lot. Are you just over the line? Are you just under the line? It’s fluctuating, the eligibility changes. But you try to do three years at once after all the chaos, with political undercurrents such as the nonexpansion states, and it makes it harder and messier.
Rovner: Which was predicted and came true. So yet another theme from this year is what I’m calling the managed care backlash redux. In the late 1990s, when lots of people were herded into managed care for the first time, there were lots of horror stories about patients being denied care, doctors being put through bureaucratic hoops, unqualified people making medical decisions. There’s a bipartisan bill that almost came to fruition in 2001 for what was called a patient’s bill of rights, but it was pushed off the agenda by 9/11. Most of the protections in that bill, however, were eventually included in the Affordable Care Act.
So now it’s 2023, and lo and behold, those same issues are back. A top issue for the American Medical Association this year is reining in prior authorization requirements, which require doctors to actually get permission before their patients can get recommended care. In one particularly painful story recently, a woman who’d been approved for a lung transplant had her surgery canceled by her insurer, literally on the way to the OR [operating room]. Later, and not coincidentally after a public outcry, the insurer, Cigna, called the whole thing, quote, “An error.” So she did finally get her lung transplant. Joanne, you covered the patient’s bill of rights fight with me back in the day. Most things that are being complained about now are now illegal. So why are we seeing so much of it again?
Kenen: Because there’s confusion about — patients don’t know what their rights are. All of us are savvy and all of us have had something in our own insurance that we don’t understand, or maybe we end up navigating it, but it’s not ever easy. Things like prior authorization — they say, “Well, we have to make sure people are getting appropriate care.” There is an element of truth there; there is overuse in American health care. There are people who get things they don’t really need or should try something less intrusive and less expensive first. So you have this genuine issue of overtreatment, back surgery being the classic example. Many people will do just as well with physical therapy and things like that than they will with an $80,000 operation. In fact, they might do better with the PT and not with the $80,000 operation.
So is there any validity to the idea of making sure people get appropriate care? Yes, but they say no to stuff that they should be covering. That’s clear, and that patients don’t always know what the right pathway is, because doctors also have incentives, or just the way they’re trained and the way they look at their — surgeons like to cut. It’s what they’re trained to do. They trained for years. So it’s really complicated, because there’s this collision between overuse and overtreatment and overcharging and all the over, over, over stuff that comes from the provider world and the no, no, no, no, no, no, no, “you can’t have that” stuff that comes from the insurer world, sometimes appropriately, but often not appropriately.
Rovner: Then I guess you load onto that the private equity and now the providers whose overlords are in it to make a profit. Then you have sort of private equity butting heads with insurance, which is one of the reasons I think we are sort of ending up here. But it certainly does feel very reminiscent of things that I’ve been through before. We’re seeing yet a similar story with Medicare Advantage, which is the private Medicare managed care program that now enrolls more than half of the Medicare population and makes lots of money for its private insurance companies that offer them.
Rachel, your colleagues wrote about a Humana algorithm that was being used to deny care after a patient had received it for, quote-unquote, “an average period of time, regardless of the patient’s condition,” meaning that if patient is sicker than average, they were saying, “Too bad, we’re only going to give this to you for 18 days because that’s what the average patient needs. If you need more, sorry about that.” So Congress is now trying to get into the act, trying to ensure that Medicare patients, who tend to vote in disproportionate numbers, get their needed care. The insurance industry is pushing back against the pushback. What’s the outlook for Congress actually getting something done on this issue? I’ve heard a lot of talk. I haven’t seen a whole lot of action.
Cohrs: Yeah, I mean certainly there has been talk — and just to point out that the Humana lawsuit is related to the UnitedHealth Group lawsuit that we saw earlier; it’s the same company making the algorithm. Bob and Casey’s reporting was just more focused on UnitedHealth Group, because they got internal documents showing the correlation between the quote-unquote “recommendation” of this algorithm and care decisions and denials and people being cut off from their rehab services. So I think certainly, I think there has been a lot of outcry. We’re seeing this play out in the legal system beforehand. This is an issue that we’ve discussed as well.
Are we going to regulate through the courts, because everything else is too slow? I think AI is certainly a hot topic on the Hill at the moment, and there is lawmaker interest, but this is just a very complicated space. Lawmakers, though they might try their best, are not the most tech-savvy people. These are very powerful interests that I would imagine would oppose some of these regulations if they were to actually materialize. So, there’s nothing imminent. Certainly if we see these lawsuits keep piling up, if we see discovery, if we see some more examples of this happening where other companies are using the algorithms as well, a groundswell — as you mentioned, Medicare patients are an important constituency — I think we could see some action, but it’s not looking imminent at this time.
Kenen: The other thing is there’s been a number of reports from a number of media outlets, Stat and others, that these algorithms are being used without any people to work with them. Like, OK, here’s this algorithm and it’s doing these batches of like, I’m going to say no to 50,000 people in 20 seconds. I’m exaggerating a little bit there, but yes, is there legitimate questions about what is appropriate treatment? Yes.
Or you hear these stories about people told, “You can’t have this drug; you have to have that drug at first,” but they would try that drug and it didn’t work for them, and there’s just no way of — the reason we have five or six similar drugs is that in some cases, those slight differences, people respond differently, mental health being a huge example of that, right? Where it could be very hard to get people on the right drugs, if person A doesn’t respond the same way as person B, even if they have the same condition. But 50,000, I don’t know if that’s the right number, but I think I remember reading one where it was 50,000 going through an algorithm. That’s not appropriate use; that’s mass production of saying no to some legitimate needs.
Rovner: Sandhya, I see you nodding there. I know that this is something that’s kind of bipartisan, right? Members of Congress get complaints about Medicare, which is something that they do, members of Congress, oversee. It is a government program, even though these are being run by private companies. I’m sort of wondering when this is going to reach a boiling point that’s going to require something to be done.
Raman: I think with some of these issues that we face that are kind of evergreen here, there has been a bipartisan push to find kind of ways to reform the prior authorization process. We’ve had people as different as Sen. Elizabeth Warren (D-Mass.) and Sen. Mike Crapo (R-Idaho) say they want reform, or Sen. James Lankford (R-Okla.) is very different from Rep. Pramila Jayapal (D-Wash.), and they’ve both said that, similar things that …
Rovner: Some of the most conservative and the most liberal members of Congress.
Raman: Yeah, so we’ve got a broad stretch, but I think at the same time, if you look at some of the other things that we have to deal with here — Congress is out for the year, but for next year, we are fairly behind in that we have a long list of things that need to be extended by mid-January. Then we have just funding all of HHS and a number of other government things by early February. So getting something from start to finish next year, which is also an election year, is going to be tough. So I think that there’s interest there, but I don’t know that getting something hashed out is going to be the easiest next year of all years.
Rovner: Yeah, I think it’s fair to say that Congress took an incomplete in most subjects this year. Well, finally this week, the topic that I think has been in every podcast this year, which is abortion. One of the threads that has wound through this year’s coverage is the strong support for abortion rights from voters, even in red and red-ish states. This year, Ohio voters affirmed a right to abortion, twice actually; there was a technical vote back in the summer. And in Virginia, Democrats flipped the legislature by running against Republican promises to impose a compromise 15-week ban, which apparently did not seem to be a compromise to most of the voters. That was after a half a dozen states voted in favor of the abortion rights position in the 2022 midterms. So this week we have a pair of stories, one from Politico and one from The New York Times, about how anti-abortion forces are working to keep future abortion-related questions off of the ballot in states where there’s still that possibility, including Florida, Missouri, Arizona, and Nevada.
One Republican Missouri lawmaker said that the right to life, quote, “should not be taken away because of a vote by a simple majority,” which frankly felt a little breathtaking to me. He has filed a bill that would require ballot measures to pass not just statewide, but with a majority in more than half of the state’s congressional districts. So basically in the really red parts of the states, a majority there would also have to vote for this. These people are getting very creative in their attempts to stop these votes from happening, maybe because they don’t think they can win them if it’s just straight up or down.
Raman: I think one thing to look at is kind of how we see some of these similar tactics in the same way that we saw with Medicaid. When Medicaid expansion started winning on different ballots, there were states that tried to put in measures to kind of tamp that down, saying, “You need a higher threshold,” and maybe that doesn’t pass, but still putting in different tactics to reduce the likelihood of that passing. I think that’s kind of what we’ve been seeing here, whether or not it’s Ohio trying to change its threshold, or we’ve had states say that even if something passed, let’s try to tear that back so that it doesn’t actually get implemented, or ahead of the ones for next year, let us find tactics to reduce the likelihood they’ll get the signatures to be on the ballot or reduce the likelihood of it passing by changing the language or pushing for challenging the language.
So there’s kind of what we saw right after the Dobbs decision, which was just a very “throw spaghetti at the wall, see what sticks,” just kind of ramp up things and see what will work, given that the last — all of the elections that we’ve had post-Dobbs have been in the favor of abortion rights. Even when we’ve tried to pass an anti-abortion measure, it’s not passed at the ballot. In the stories that you mentioned, there was another quote that stuck out to me, where they’d also mentioned that maybe this should not be subject to majority vote, I think in the Politico piece as well. So I think that’s something that is interesting that I haven’t really seen vocalized before, that this should be done in a different manner rather than this is how the majority of people feel one way or the other.
Rovner: Yeah, it felt so ironic because when in the Dobbs decision, Justice [Samuel] Alito wrote, “Well, now we’re turning this back to the states to be decided by their voters.” Well, here are their voters deciding, and it turns out the anti-abortion side don’t like the way the voters are voting, so they’re going to try to not have the voters vote, basically. We will see how this one all plays out. The other continuing story this year is women being prosecuted basically for bad pregnancy outcomes. Last week we talked about the case of Brittany Watts, an Ohio woman who was sent home from a hospital emergency room twice, had a miscarriage, and this week had formal charges filed against her for, quote, “abusing a corpse.” This case hasn’t gotten nearly the attention of the case of Kate Cox, the Texas woman whose fetus was diagnosed with fatal defects and who filed suit to be allowed to have an abortion.
She eventually had to go to another state, and that was even before the permission that had been granted by a lower-court judge was overturned by the Texas Supreme Court. It may be at least in part because Brittany Watts is black, or that she didn’t put herself out in public the way Kate Cox did, but this is a way that prosecutors can punish women even in states where abortion remains legal. Remember Ohio voted twice this year to keep abortion legal, and this wasn’t even an abortion; it was a miscarriage. The medical examiner determined that the fetus was already dead when it passed. What are the prosecutors trying to do here? We talk about chilling effects. This is kind of the ultimate chilling effect, right?
Raman: It really is, because here we have someone that was not, as you said, seeking an abortion. She miscarried, and I think that she was 21 weeks and five days pregnant, and then they had the 21-week cutoff. So it gets sent into really murky waters here because I’m not sure what they’re going for, kind of picking this case to prosecute and go with. We’ve had this happen before where people have self-managed or miscarried, and then they’ve ended up being prosecuted. But at this point, I’m not sure why they’re making a case out of this particular woman, kind of dragging this into the debate.
Rovner: Yeah, there was a famous case in Indiana — 2013, may have been even before that — a pregnant woman who tried to kill herself and failed to kill herself, but did kill her fetus, and she was put in jail for several years. There have been, at least there was sort of the question there, were you trying to self-abort at that point? But there was nothing here. This was a woman with a wanted pregnancy whose pregnancy ended via natural circumstances, which happens, I think we’ve discovered now, a lot more than people realize.
I think people don’t talk about unhappy pregnancy outcomes, so people don’t realize how common they actually are. But I wonder — and I’ve been saying this all year — again, if women are fearing prosecution, even women who want babies, they may fear getting pregnant. I’ve seen some stories about more permanent types of birth control happening because women don’t want to get pregnant, because they don’t want to end up in a place where their health is being risked or they’re trying to get health care they need and their doctor or they could be facing prison time.
Kenen: And in this case, she had gone to the hospital. It’s complicated. She went in and out of the hospital. She went to the ER; they sent her home. I think then once they sent her home another time, she left against medical advice, but she wasn’t trying to get an abortion. She was having pregnancy complications. It’s documented. She was in and out of medical care. Pregnancies can fail, and early, the first trimester, it’s a very high rate. It’s less common later on, but it still happens. There are times when an early miscarriage, you might not even know that it’s a miscarriage. It’s early. You don’t know what’s even going on with your own body, or you’re not certain. So she didn’t know what to do at home when she did miscarry. It seems very punitive. Did she behave in an absolutely ideal, textbook-perfect, the way you wish she might have? But she did what she could do at the time.
Rovner: Yeah, it’s hard to know what to do. Well, we will watch this case, I think, even though it’s not, as I say, it’s not getting quite the attention of some of the other cases. Our final this week in health information of 2023 goes to an ad that came to my email from the All Family Pharmacy in Boca Raton, Florida. The headline is “Miracle Drug Ivermectin for Covid-19 Could Save Lives,” and it claims that, quote, “a growing body of evidence from dozens of studies worldwide demonstrates ivermectin’s unique and highly potent ability to inhibit SARS-CoV-2 replication and aid in the recovery from covid-19.”
That sounded not quite right to me, so I looked up some of the studies that they cited and found that most had been thoroughly debunked, that ivermectin is not really good treatment for covid-19. I even found one study from an open-access journal that had to publish a correction, noting that two of its authors were paid consultants to ivermectin manufacturers, though they had failed to disclose that conflict. Meanwhile, if you don’t want ivermectin or hydroxychloroquine, which the All Family Pharmacy also sells, they will also sell you semaglutide, which is the scientific name of the hard-to-get weight loss drug Ozempic. And they say their price even includes a doctor consult. I will post the links in the show notes. All right, that is this week’s news. Now we will play my interview with Jordan Rau about his long-term care financing series. Then we’ll come back with our extra credits.
I am pleased to welcome to the podcast my KFF Health News colleague Jordan Rau. I asked Jordan to join us to talk about his latest project, “Dying Broke,” done in partnership with The New York Times. It’s about the growing expense of long-term care and the declining ability of Americans to pay for it. Jordan, welcome to “What the Health?”
Jordan Rau: Glad to be here, Julie.
Rovner: So I want you to start with the 30-second elevator pitch about what you found working on this, for two years?
Rau: Just about. The big-picture view is that when you’re elderly, if you need long-term care, by which we’re talking about nonmedical things, like personal aides, if you need help in your daily activities going to the bathroom or eating or such, or if you have a cognitive impairment like dementia, it’s exceedingly expensive, except if you are destitute. The private market solutions, which are long-term care insurance, really don’t work, and most people don’t hold it. The government solution, which is Medicaid, is only available to you once you’ve exhausted just about all of your assets and have very low income. And that’s led the vast majority of people out on their own financially to either rely on themselves or their family or other people to take up the burden. And that burden is significant for the children of older people.
Rovner: So it’s not just nursing home care that costs more than all but the richest can afford; assisted living and home care, which people assume are going to be a lot cheaper and that maybe their retirement savings will cover — they’re also increasingly out of reach. Why has the price of long-term care gone up so much faster than Americans’ retirement savings?
Rau: All of medical inflation has gone up enormously, but I think a lot of it is that there’s so little regulation on prices. There’s frankly no regulation on prices of assisted living, and you don’t have a large payer that can control prices. That’s one of the good things about Medicare, is that they set their own prices and that’s helped keep prices down. That’s why it’s less expensive for Medicare to send someone to a nursing home than for someone to pay out-of-pocket. But there’s none of that. So the prices have just gone where they’ve gone, and now you have a scarcity of workers as well. So that’s driving up wages.
Rovner: People who’ve been socking away money and thinking they’re going to be able to pay for this themselves get kind of a rude awakening when they need, and it’s not — as you say, it’s not even medical long-term care; it’s just help with activities of daily living.
Rau: Yeah, yeah, yeah. I think one of the problems is that people assume they have the best-case scenario when they’re envisioning their retirement. They’re going to be off golfing, they’re going to be playing around with their grandkids, they’re going to be taking trips. The fact is, you’re very likely — if you live well into your 80s and 90s, as many people do — to not be able to live independently anymore, to need help with at least a little bit of things, and in worst-case scenario everything. People just don’t expect that that’s going to happen.
Rovner: So why do so many Americans still not know that Medicare doesn’t pay for long-term care? I feel like I’ve been saying this since 1980-something.
Rau: I wonder how much of it would’ve been different if they had decided to name Medicaid something that isn’t so close to Medicare. Maybe that would’ve helped, but realistically, everyone I think has a sense. Well, first of all, who’s paying attention to this stuff when you’re in your 30s and 40s, right? You’re not thinking about what’s going to happen to you in the 60s. And then I think that people just don’t expect that this is going to happen to them, and Medicare has a well-earned reputation as being pretty comprehensive. It doesn’t cover certain things, and there is a “donut hole” situation, so you’ve got to get supplemental. But people know that for the most part, it’s covered. And people don’t understand that long-term care, the nonmedical side, is — not just here, everywhere — it’s the backwater of health care. It’s not even considered health care in some ways.
So you just assume — I mean, I would assume, right, if Medicare is going to cover my heart transplant, why would I not think that it’s going to cover someone to come to my house a couple hours a day to help me with stuff or to put me in an assisted living facility if it covers nursing home care? It’s such a complicated, Byzantine system. You and I, we’ve been doing this probably combined, well, I don’t want to say how long, but it’s been a long time, and it’s hard for us to untangle exactly what is covered and what overlaps with what and what are the eligibility rules. So to expect a regular person, who isn’t paid to do this 50 hours a week, to know it is highly unrealistic.
Rovner: Yeah, and I was going to say the fact that Medicare actually has a home care benefit and it has a nursing home benefit; they’re just super limited. I think that sort of adds to the confusion too, doesn’t it?
Rau: Yeah. Well, even Medicare is confused about its home care benefit, right? There’s the whole Jimmo case and a whole debate about what you need to qualify for it.
Rovner: So listeners will know that long-term care and our country’s complete lack of a long-term care policy is a pet issue of mine and has been since I started writing about it in 1986. It isn’t like the government hasn’t tried to do something. There was the ill-fated Medicare Catastrophic Coverage Act in 1988 that ended up getting repealed. There were efforts to subsidize private long-term care insurance in the 1990s that didn’t really go anywhere, and there was the CLASS [Community Living Assistance Services and Supports] Act that was briefly part of the Affordable Care Act when it passed in 2010, only to be abandoned as financially unfeasible. Why has this been such a hard issue to address from a policy point of view?
Rau: The one-word answer obviously is money. It’s incredibly expensive. So to have that type of lift, it would be to expand either Medicaid or Medicare or to create a new program; would be inordinately expensive. But beyond that, I think basically, to do this, you either have to tag on something to one of those existing programs, which is a major expansion, or you have to have a mandatory insurance program. It could be a public one; it can be a private one. I think that it’s hard because it’s not universal. Auto insurance — everybody drives, right? So if you say, OK, you all know you’re going to drive, and people know like, Oh, I may get into an accident. So then you have a functioning insurance market.
Health insurance, sort of the same thing. Everyone knows that they’re going to need health insurance maybe next year. So that’s an easier sell. Even that, right, with the Affordable Care Act — that passed by just one vote. That was a heavy lift. So here you’re saying, here’s something that you may need but you very well may never tap. By the way, we want you to pay for it now or buy into it now, and it’s not relevant for your life until 30 years. I just think that’s a hard sell politically to the population, to the political system. It’s a hard sell.
Rovner: So if there was just one message that you hope people take away after reading this exhaustive series, what do you think it should be?
Rau: Printing the series out and frame it and put it on your wall would be my main message. But I would say that this stuff is so unpredictable that you really have to have some flexibility in your expectations and planning, because you can’t plan to not get early-onset dementia. You can’t plan to need help. So I think that you need to — people obviously need to have as much of a cash cushion as they can, and they need to bone up on this before it’s a crisis, because by the time it’s a crisis — and this is a problem, right, with health insurance too. By the time you’ve got the emotional and health issues, to throw on top of it a bureaucratic sort of financial issue is just so hard for most people to juggle. So there isn’t an easy solution, but it is important for people to realize that this is as much of a risk as smashing your car into a telephone pole and that you cannot have one answer.
Your answer cannot be like, “Oh, well I’m just going to stay in my house, because you may not be able to stay in your house.” Or your answer can’t be, “Well, I’m going to go into a fancy assisted living facility with a great chandelier and great food,” because unless you save an inordinate amount of money, even if you go in there, you may not be able to afford to stay there. So it’s really a recognition that you can’t really concretely plan for this, but you may very well not be able to live independently if you are lucky enough to live into your eighth and ninth decade.
Rovner: Great. Jordan Rau, anything I didn’t ask?
Rau: Never. Never, Julie.
Rovner: Jordan Rau, thank you so much for joining us.
Rau: Great to see you.
Rovner: OK. We are back, and it’s time for our last extra credit segment of the year. That’s when we each recommend a story we read this week we think you should read too. As always, don’t worry if you miss it. We will post the links on the podcast page at kffhealthnews.org and in our show notes on your phone or other mobile device. Rachel, why don’t you go first this week?
Cohrs: Sure. The story I chose is in ProPublica. The headline is “Doctors With Histories of Big Malpractice Settlements Work for Insurers, Deciding If They’ll Pay for Care,” by Patrick Rucker at The Capitol Forum and David Armstrong and Doris Burke at ProPublica. I think this article very much fits into the larger theme we were talking about earlier about insurance denials. This was pretty shocking still to me, of these instances of doctors with big malpractice settlements that had been disciplined by medical boards failing up essentially and getting jobs. If they can’t practice anymore, then they’re getting jobs in insurance companies instead, deciding whether a much larger volume of patients get care. So I think it was just a fascinating, really well-done investigation. It sounded like it was really difficult to match up all the records with the lawsuits and the settlements, and there aren’t necessarily databases that exist of what doctors work for insurance companies. So it was just really well done and just a really important space that we’ll continue to talk about.
Kenen: That was a great piece. These doctors are making $300,000 to $400,000 a year, these people who failed up, as Rachel just put it. Yeah.
Rovner: Yeah. That’s the perfect phrase. Sandhya.
Raman: My extra credit this week is called “Mississippi Community Workers Battle Maternal Mortality Crisis,” and it’s from my colleague at Roll Call Lauren Clason. This story also illustrates a combination of themes from this year. It touches on some of the maternal health inequities, the racial inequities, and rural health inequities, and how politics kind of comes into all of that. Mississippi Black women die at a rate four times higher than white women, and the state also leads in infant mortality rates nationwide. At the same time, it’s also a nonexpansion state for Medicaid. So Lauren went to Mississippi to look at some of the community and state-led groups that are trying to reduce these inequities that are caused by the different racial, socioeconomic, and access factors that are happening at the same time that an increasing number of hospitals are closing in the state.
Rovner: Also another really good story. Joanne?
Kenen: The theme of the day is yearlong, or decades-long in some cases, but ongoing health stories that have dominated the year. Another one that we didn’t touch on today but clearly is an ongoing multiyear health crisis is gun violence, which is a public health problem as well as a criminal justice problem. The Trace did a fantastic end-of-year project by Justin Agrelo. It’s called “Chicago Shooting Survivors, in Their Own Words.” They worked with both people who had survived shootings as well as people who had lost family members to shootings, and they worked with them about how to write and tell stories.
These five stories are in these people’s own words, and it was partnered with a bunch of other Chicago-based publications. They’re very powerful. In the introduction, they wrote that the Chicago media has been really good about trying to cover every homicide but that these people end up being defined by their death, not everything else about their life. These essays, they didn’t just talk about grief, which is obviously a huge — grief and trauma — but also the lives, not just the deaths. It’s really, really worth spending some time with.
Rovner: Yeah, and we haven’t talked as much as we probably should have about gun violence, but we will put that on the list for 2024. My extra credit this week is from Business Insider. It’s called “I Feel Conned Into Keeping This Baby.” It’s by Bethany Dawson, Louise Ridley, and Sarah Posner. It’s about an anti-abortion group that promised pregnant women financial support for their babies if they agreed not to get an abortion. But even though the women signed contracts, the group, called Let Them Live, did not provide the aid promised. Apparently they promised more money than they could raise in contributions. Now, I have heard of pregnancy crisis centers promising things like diapers and formula, but this group said it would help with groceries and rent and other significant expenses until it didn’t. Apparently the small print in the contract said the benefits could be reduced or stopped at any time. This was supposed to help answer the criticism that anti-abortion groups don’t actually care about the women, particularly after they give birth, except maybe promising things that you can’t deliver isn’t the best way to do that.
OK. That is our show for this week and for this year. As always, if you enjoy the podcast, you can subscribe wherever you get your podcasts. We’d appreciate it if you left us a review; that helps other people find us, too. Special thanks, as always, to our technical guru, Francis Ying, and our editor, Emmarie Huetteman. Also as always, you can email us your comments or questions. We’re at whatthehealth@kff.org. Or you can still find me at X, @jrovner, or @julierovner at Bluesky and @julie.rovner at Threads. Sandhya, where are you on social media these days?
Raman: I’m @SandhyaWrites on both X and Bluesky.
Rovner: Rachel.
Cohrs: I’m @rachelcohrs on X, @rachelcohrsreporter on Threads.
Rovner: Joanne.
Kenen: @joannekenen1 on Threads. I’m occasionally on X — or, as you all know, I’ve been calling it Y — @JoanneKenen.
Rovner: We will be back in your feed in 2024. Until then, have a great holiday season, and be healthy.
Credits
Francis Ying
Audio producer
Emmarie Huetteman
Editor
To hear all our podcasts, click here.
And subscribe to KFF Health News’ “What the Health?” on Spotify, Apple Podcasts, Pocket Casts, or wherever you listen to podcasts.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 7 months ago
Health Care Costs, Medicaid, Medicare, Multimedia, Pharmaceuticals, Public Health, Abortion, Drug Costs, Hospitals, KFF Health News' 'What The Health?', Legislation, Medicare Advantage, Podcasts, Women's Health
STAT+: Patent thickets and terminal disclaimers: How pharma blocks biosimilars from the marketplace
To ring the register, a pharmaceutical company may create a patent thicket, which involves filing dozens of patents that, in some cases, add little value to their medicines but extend precious monopolies.
To ring the register, a pharmaceutical company may create a patent thicket, which involves filing dozens of patents that, in some cases, add little value to their medicines but extend precious monopolies. And one crucial, but little-known tool for making this happen is something called a terminal disclaimer.
In short, a terminal disclaimer is a stipulation provided to the U.S. Patent & Trademark Office that a continuation or follow-on patent – essentially, a minor patent that makes few substantive changes to a medicine – will expire at the same time as the original patent filed by a pharmaceutical company. By doing so, a drugmaker can circumvent prohibitions on awarding more than one patent for an invention.
As a result, a drug company can quickly add a number of patents that can be used to protect its medicines from would-be rivals. How so? As patents pile up, companies that want to sell generic or biosimilar versions of these medicines find themselves fighting longer and more expensive patent infringement lawsuits that are designed to delay their plans.
1 year 7 months ago
Pharma, Pharmalot, patents, Pharmaceuticals, STAT+
A New Test Could Save Arthritis Patients Time, Money, and Pain. But Will It Be Used?
SAN DIEGO — Erinn Maury knew Remicade wasn’t the right drug for Patti Schulte, a rheumatoid arthritis patient the physician saw at her Millersville, Maryland, practice. Schulte’s swollen, painful joints hadn’t responded to Enbrel or Humira, two drugs in the same class.
But the insurer insisted, so Schulte went on Remicade. It didn’t work either.
SAN DIEGO — Erinn Maury knew Remicade wasn’t the right drug for Patti Schulte, a rheumatoid arthritis patient the physician saw at her Millersville, Maryland, practice. Schulte’s swollen, painful joints hadn’t responded to Enbrel or Humira, two drugs in the same class.
But the insurer insisted, so Schulte went on Remicade. It didn’t work either.
What’s more, Schulte suffered a severe allergic reaction to the infusion therapy, requiring a heavy dose of prednisone, a steroid with grave side effects if used at high doses for too long.
After 18 months, her insurer finally approved Maury’s drug of choice, Orencia. By then, Schulte’s vertebrae, weakened by prednisone, had started cracking. She was only 60.
Schulte’s story of pain, drug-hopping, and insurance meddling is all too common among patients with rheumatoid arthritis, who often cycle agonizingly through half a dozen drugs in search of one that provides a measure of relief. It’s also a story of how doctors are steered by pharmacy benefit managers — the middlemen of the drug market — as well as by insurers.
Once people with inflammatory conditions such as rheumatoid arthritis reach a certain stage, the first prescription offered is typically Humira, the best-selling drug in history, and part of a class known as tumor necrosis factor inhibitors, or TNFis, which fail to significantly help about half of the patients who take it.
“We practice rheumatology without any help,” said Vibeke Strand, a rheumatologist and adjunct clinical professor at Stanford. She bemoaned the lack of tools available to choose the right drug while bristling at corporate intervention in the decision. “We are told by the insurer what to prescribe to the patient. After they fail methotrexate, it’s a TNF inhibitor, almost always Humira. And that’s not OK.”
If there’s a shred of hope in this story, it’s that a blood test, PrismRA, may herald an era of improved care for patients with rheumatoid arthritis and other autoimmune conditions. But first, it must be embraced by insurers.
PrismRA employs a predictive model that combines clinical factors, blood tests, and 19 gene patterns to identify the roughly 60% of patients who are very unlikely to respond to a TNFi drug.
Over the past 25 years, drug companies have introduced five new classes of autoimmune drugs. TNFis were the first to market, starting in the late 1990s.
Some 1.3 million Americans have rheumatoid arthritis, a disease in which a person’s immune system attacks their joints, causing crippling pain and, if improperly treated, disfigurement. The newer drugs, mostly so-called biologics, are also used by some of the 25 million or more Americans with other autoimmune diseases, such as lupus, Crohn’s disease, and psoriasis. Typically costing tens of thousands of dollars annually, the drugs are prescribed after a patient fails to respond to older, cheaper drugs like methotrexate.
Until recently, rheumatologists have had few ways to predict which of the new drugs would work best on which patients. Often, “it’s a coin flip whether I prescribe drug A or B,” said Jeffrey Curtis, a rheumatology professor at the University of Alabama-Birmingham.
Yet about 90% of the patients who are given one of these advanced drugs start on a TNFi, although there’s often no reason to think a TNFi will work better than another type.
Under these puzzling circumstances, it’s often the insurer rather than the doctor who chooses the patient’s drug. Insurers lean toward TNFis such as adalimumab, commonly sold as brand-name Humira, in part because they get large rebates from manufacturers for using them. Although the size of such payments is a trade secret, AbbVie is said to be offering rebates to insurers of up to 60% of Humira’s price. That has enabled it to control 98.5% of the U.S. adalimumab market, even though it has eight biosimilar competitors.
PrismRA’s developer, Scipher Medicine, has provided more than 26,000 test results, rarely covered by insurance. But on Oct. 15, the Centers for Medicare & Medicaid began reimbursing for the test, and its use is expected to rise. At least two other companies are developing drug-matching tests for rheumatoid arthritis patients.
Although critics say PrismRA is not always useful, it is likely to be the first in a series of diagnostics anticipated over the next decade that could reduce the time that autoimmune disease patients suffer on the wrong drug.
Academics, small biotechs, and large pharmaceutical companies are investing in methods to distinguish the biological pathways involved in these diseases, and the best way to treat each one. This approach, called precision medicine, has existed for years in cancer medicine, in which it’s routine to test the genetics of patients’ tumors to determine the appropriate drug treatment.
“You wouldn’t give Herceptin to a breast cancer patient without knowing whether her tumor was HER2-positive,” said Costantino Pitzalis, a rheumatology professor at the William Harvey Research Institute in London. He was speaking before a well-attended session at an American College of Rheumatology conference in San Diego in November. “Why do we not use biopsies or seek molecular markers in rheumatoid arthritis?”
It’s not only patients and doctors who have a stake in which drugs work best for a given person.
When Remicade failed and Schulte waited for the insurer to approve Orencia, she insisted on keeping her job as an accountant. But as her prednisone-related spinal problems worsened, Schulte was forced to retire, go on Medicaid, and seek disability, something she had always sworn to avoid.
Now taxpayers, rather than the insurer, are covering Schulte’s medical bills, Maury noted.
Precision medicine hasn’t seemed like a priority for large makers of autoimmune drugs, which presumably have some knowledge of which patients are most likely to benefit from their drugs, since they have tested and sold millions of doses over the years. By offering rebate incentives to insurers, companies like AbbVie, which makes Humira, can guarantee theirs are the drugs of choice with insurers.
“If you were AbbVie,” Curtis said, “why would you ever want to publish data showing who’s not going to do well on your drug, if, in the absence of the test, everyone will start with your drug first?”
What Testing Could Do
Medicare and commercial insurers haven’t yet set a price for PrismRA, but it could save insurers thousands of dollars a year for each patient it helps, according to Krishna Patel, Scipher’s associate director of medical affairs.
“If the test cost $750, I still only need it once, and it costs less than a month of whatever drug is not going to work very well for you,” said Curtis, a co-author of some studies of the test. “The economics of a biomarker that’s anything but worthless is pretty favorable because our biologics and targeted drugs are so expensive.”
Patients are enthusiastic about the test because so many have had to take TNFis that didn’t work. Many insurers require patients to try a second TNFi, and sometimes a third.
Jen Weaver, a patient advocate and mother of three, got little benefit from hydroxychloroquine, sulfasalazine, methotrexate, and Orencia, a non-TNFi biologic therapy, before finding some relief in another, Actemra. But she was taken off that drug when her white blood cells plunged, and the next three drugs she tried — all TNFis — caused allergic reactions, culminating with an outbreak of pus-filled sores. Another drug, Otezla, eventually seemed to help heal the sores, and she’s been stable on it since in combination with methotrexate, Weaver said.
“What is needed is to substantially shorten this trial-and-error period for patients,” said Shilpa Venkatachalam, herself a patient and the director of research operations at the Global Healthy Living Foundation. “There’s a lot of anxiety and frustration, weeks in pain wondering whether a drug is going to work for you and what to do if it doesn’t.” A survey by her group found that 91% of patients worried their medications would stop working. And there is evidence that the longer it takes to resolve arthritis symptoms, the less chance they will ever stop.
How insurers will respond to the availability of tests isn’t clear, partly because the arrival of new biosimilar drugs — essentially generic versions — are making TNFis cheaper for insurance plans. While Humira still dominates, AbbVie has increased rebates to insurers, in effect lowering its cost. Lower prices make the PrismRA test less appealing to insurers, since widespread use of the test could cut TNFi prescriptions by up to a third.
However, rheumatologist John Boone in Louisville, Kentucky, found to his surprise that insurers mostly accepted alternative prescriptions for 41 patients whom the test showed unlikely to respond to TNFis as part of a clinical trial. Boone receives consulting fees from Scipher.
Although the test didn’t guarantee good outcomes, he said, the few patients given TNFis despite the test results almost all did poorly on that regimen.
Scientists from AbbVie, which makes several rheumatology drugs in addition to Humira, presented a study at the San Diego conference examining biomarkers that might show which patients would respond to Rinvoq, a new immune-suppressing drug in a class known as the JAK inhibitors. When asked about its use of precision medicine, AbbVie declined to comment.
Over two decades, Humira has been a blockbuster drug for AbbVie. The company sold more than $3.5 billion worth of Humira in the third quarter of 2023, 36% less than a year ago. Sales of Rinvoq, which AbbVie is marketing as a treatment for patients failed by Humira and its class, jumped 60% to $1.1 billion.
What Patients Want
Shannan O’Hara-Levi, a 38-year-old in Monroe, New York, has been on scores of drugs and supplements since being diagnosed with juvenile arthritis at age 3. She’s been nauseated, fatigued, and short of breath and has suffered allergic reactions, but she says the worst part of it was finding a drug that worked and then losing access because of insurance. This happened shortly after she gave birth to a daughter in 2022, and then endured intense joint pain.
“If I could take a blood test that tells me not to waste months or years of my life — absolutely,” she said. “If I could have started my current drug last fall and saved many months of not being able to engage with my baby on the floor — absolutely.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 7 months ago
Health Care Costs, Health Industry, Pharmaceuticals, Autoimmune Diseases, Drug Costs, Prescription Drugs
The Market for Biosimilars Is Funky. The Industry Thinks PBMs Are To Blame
Over the past year there’s been movement to rein in the three big PBMs, which face little regulation though they help set drug prices and drug choices for 80 percent of Americans and their doctors.
The House voted Dec. 11, 320-71, for legislation that would require the PBMs to change some of the ways they do business. The big three — CVS Health, Express Scripts, and OptumRx — have all announced their own reform measures in recent months.
The bill looks unlikely to pass the Senate, though some of its provisions might eventually become law. Meanwhile, some of the most baffling contradictions of PBM drug pricing are coming to a head.
Take AbbVie’s Humira, the highest-earning drug ever. Eight biosimilars — what ordinary people would call generics — came onto the market this year, raising hopes of big savings for patients and insurers. Some cost as little as $995 a month, compared to Humira’s wholesale price of $6,992.
The Pharmaceutical Care Management Association, which represents the big PBMs, told KFF Health News in a statement that its members are pushing to use more of the biosimilars. Why then, asks Juliana Reed, CEO of the Biosimilars Forum trade group, did Humira account for 98.5 percent of all sales of the drug and its biosimilars as recently as November?
I’ve been told that AbbVie has threatened to withhold rebates it pays PBMs for some of its other medicines unless they give Humira good placement on formularies, the all-important lists of drugs available to their customers. The PBMs say their formularies provide the best deal for employers, but these are “assertions impossible to verify,” says James Gelfand, president and CEO of The ERISA Industry Committee, which represents large employers.
The PBMs’ strategy is purposefully obscure. Negotiations with drugmakers constitute their special sauce and they aren’t sharing the ingredients. But given that it costs up to $300 million to develop a biosimilar, the Humira battle is key to the future of biosimilars in general, and to more competition to lower expensive drug prices.
“If you can’t break into anti-inflammatory drugs it will be hard to break into any model,” Gelfand said. “It’s the weather vane, the shape of things to come.”
There’s more weird stuff going on with biosimilars. To get Inflectra, its biosimilar to Johnson & Johnson’s blockbuster Remicade, onto formularies, Pfizer pays large rebates to insurers, I’ve been told.
That’s driven down average net prices for Inflectra as well as other versions of the drug.
Good, right? Not according to rheumatologists, the doctors who typically administer these complicated, infused drugs in their offices.
The doctors say they still have to pay much higher prices to obtain Inflectra from distributors. But their reimbursement from Medicare is reduced because of the rebates, they say. Several rheumatologists told me that the way the math works out — or rather, doesn’t — they could lose as much as $20,000 a year on each patient.
The choice is “lose money, or divert the patient to a hospital infusion center,” said Chris Phillips, a doctor in Paducah, Ky., who chairs the American College of Rheumatology’s insurance subcommittee. The latter is “more expensive and usually not as good an experience for the patient.”
Payment imbalances also have developed for Amgen’s Avsola, another Remicade biosimilar, and for biosimilar forms of Genentech’s Rituxan, a cancer drug also infused to treat autoimmune conditions, rheumatologists say.
“The whole point of biosimilars is to make these drugs more accessible, but they’re becoming unaffordable,” said Madelaine Feldman, immediate past president of the Coalition of State Rheumatology Organizations.
Spokespeople for Pfizer and for the Pharmaceutical Care Management Association acknowledged the rheumatologists’ dilemma. Each said it was up to the other to resolve the problem.
This article is not available for syndication due to republishing restrictions. If you have questions about the availability of this or other content for republication, please contact NewsWeb@kff.org.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 7 months ago
Health Care Costs, Health Industry, Pharmaceuticals, Prescription Drugs, The Health 202
STAT+: Here are the best biopharma CEOs of 2023
It’s that time of the year again when I recognize the Best Biopharma CEO of the year.
This year’s selection is so deservingly obvious that I won’t fabricate suspense by starting with an honor roll of runners-up. More on those high-achieving folks later. Let’s get right to the main course: David Ricks of Eli Lilly is the runaway, rock star, who-else-could-it-be Best Biopharma CEO of 2023.
What an incredible year it’s been for Ricks and the Lilly executive team who helped him achieve so much. My colleague Matt Herper wrote earlier this year about the “dynamic duo” of Ricks and Chief Scientific Officer Dan Skovronsky.
1 year 7 months ago
Adam's Take, Biotech, biotechnology, Eli Lilly, Novo Nordisk, Pharmaceuticals, STAT+
Millions of Dollars Flow From Pharma to Patient Advocacy Groups
Pharma money is all over the place — in universities, companies doing continuing medical education for doctors and in prominent patient advocacy organizations that are household names across America.
Public Citizen, a consumer advocacy nonprofit, reports today that between 2010 and 2022, the drug industry’s main lobbying group and member companies provided at least $6 billion in grants to more than 20,000 organizations. The analysis, provided exclusively to KFF Health News in advance of its release, focused on the Pharmaceutical Research and Manufacturers of America (PhRMA) and 31 drug companies that were members of the trade group as of March.
The money dwarfs industry spending over that time on federal lobbying and campaign contributions to lawmakers. With high drug prices a regular topic of debate in Washington, drug industry grants to patient advocacy groups in particular raises questions about conflicts of interest — including whether organizations that accept the industry’s money shy away from pushing policies the drugmakers oppose, even if patients may benefit.
“There’s a risk that those entanglements influence the work of those organizations,” said Matthew McCoy, an assistant professor of medical ethics and health policy at the University of Pennsylvania who has studied patient advocacy groups’ influence and transparency.
He said there’s another important dynamic likely at play, too. Companies aren’t blindly choosing which groups to fund but instead are “probably selecting organizations that are already inclined to see the world, see the policy issues, the way they see it.”
A couple examples you’ll recognize: The American Heart Association received $64.1 million over the 12-year period. The American Cancer Society and its advocacy affiliate, the American Cancer Society Cancer Action Network, together received $23.1 million.
The question is whether the money affects the heavyweight groups’ advocacy. After the House passed the Inflation Reduction Act in August 2022, the American Cancer Society Cancer Action Network’s statement hailed the bill’s cap on Medicare enrollees’ out-of-pocket costs for prescription drugs and additional tax credits for ACA insurance plans. But the group was silent on a contentious provision giving Medicare the ability to negotiate drug prices with manufacturers.
Lisa Lacasse, president of the American Cancer Society Cancer Action Network, said in an emailed statement that the organization didn’t take a formal position on Medicare drug negotiation because “the policy’s impact on patient access to and affordability of cancer treatments was unclear.” In contrast, the Part D out-of-pocket cap “has evidence-based patient benefit.”
- “ACS CAN’s only constituents are cancer patients, survivors, and their loved ones nationwide. ACS CAN’s policy agenda is driven entirely by evidence with the single purpose of achieving our mission to end cancer as we know it, for everyone,” she added. “Contributions to the organization do not influence policy decisions or positions.”
The American Heart Association similarly has touted its support of the three-year extension of enhanced Obamacare tax credits in the IRA, but was silent on drug price negotiation. “We have strict standards in place to monitor relationships with industry and protect against conflicts of interest,” Steve Weiss, a spokesperson for the group, said in an emailed statement. “These funds in no way influence our advocacy, programs or science.”
- “We engage with different organizations who have a wide array of health care opinions and priorities,” Alex Schriver, senior vice president of public affairs at PhRMA, said in an emailed statement. “We may not agree on every issue, but we believe engagement and dialogue is important to promoting a health care policy environment that supports innovation, a highly-skilled workforce and access to lifesaving medicines.”
While the patient groups’ primary mission is to advocate for people with particular diseases, including by boosting funding for research, their work in Washington often bolsters that of pharmaceutical companies whose drugs their patients rely upon. At the same time, the story isn’t always black and white, and just because a group gets money doesn’t automatically make them a “pharma lackey,” said Mike Tanglis, research director at Public Citizen. “It’s not so clean cut,” McCoy added.
The American Diabetes Association received $26.4 million from the drug industry — yet the group supported allowing Medicare to negotiate prescription drug prices. Multiple drug companies have sued to stop the program.
Francisco Prieto, chair of the American Diabetes Association’s national advocacy committee, said in a statement that support from its corporate and other partners allows the group to provide resources about diabetes to health care personnel as well as patients and their caregivers.
“Our partners do not influence our business or policy decisions, which are made solely based on our mission and what is in the best interest of patients around the world,” he said.
Many groups receiving grants do criticize high drug prices generally or highlight patients’ difficulties in affording care. But, “specifically calling out pharma companies doesn’t seem to be a huge priority for them,” Tanglis said.
It isn’t always obvious which groups drug companies are paying. Congress in 2010 enacted the Physician Payments Sunshine Act, a law that required payments to physicians from drug and medical device makers to be registered on a public website. But patient groups were not addressed in the bill. Drug companies’ payments to patient groups can be — but aren’t always — included in annual filings to the IRS or in charitable giving reports.
The American Heart Association in its 2022 annual report lists contributions from corporations, foundations and others. The American Diabetes Association in its latest annual report lists corporate sponsors and ranges for the amounts they give, but not precise dollar figures. Similarly, the American Cancer Society’s most recent report names corporate sponsors giving more than $1 million, but exact amounts aren’t disclosed.
McCoy believes there should be “some kind of mandated transparency across the board” for payments and that patient groups should openly answer questions about steps they take to make sure the funding doesn’t influence their decisions.
“Those are all great things that patient advocacy groups can and should be doing,” McCoy said.
This article is not available for syndication due to republishing restrictions. If you have questions about the availability of this or other content for republication, please contact NewsWeb@kff.org.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 7 months ago
Health Industry, Pharmaceuticals, The Health 202