AbbVie’s $2.1B Acquisition Adds In Vivo Cell Therapy to Its Immunology & Inflammation Pipeline
3 months 4 weeks ago
BioPharma, Pharma, AbbVie, autoimmune disease, CAR-T, cell theapy, Clinical Trials, immunology, inflammation
Metsera’s Amylin Drug Looks Good in Phase 1, Shows Potential to be a Once-Monthly Obesity Med
4 months 2 weeks ago
BioPharma, Pharma, amylin, Clinical Trials, GLP-1 drugs, metabolic disorder, Metsera
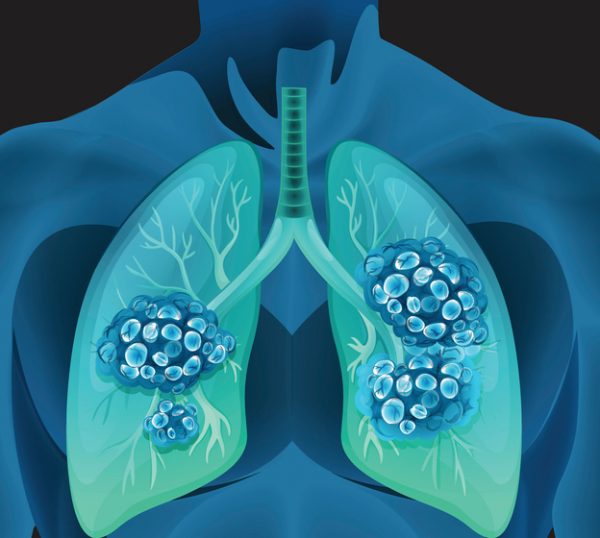
AbbVie’s Solid Tumor Strategy Gets a Win With Accelerated FDA Approval in Lung Cancer
5 months 2 weeks ago
BioPharma, Daily, legal, Pharma, AbbVie, antibody drug conjugate, biopharma nl, Clinical Trials, Emrelis, FDA, lung cancer
STAT+: In Ireland, a global hub for the pharma industry, Trump tariffs are a source of deep worry
6 months 1 week ago
Biotech, Pharma, Pharmaceuticals, policy, STAT+
NIH director targets misinformation research as more turmoil rocks health agencies
7 months 3 days ago
Biotech, Business, Pharma, The Readout, biotechnology, drug development, drug prices, Research
STAT+: AbbVie, J&J to add proprietary data to AI protein model in bid to accelerate drug discovery
7 months 3 days ago
Biotech, Health Tech, Pharma, Artificial Intelligence, Health Tech, STAT+
Altis says its AI tool can cut risk in cancer trials
7 months 4 days ago
Biotech, Business, Pharma, The Readout, biotechnology, drug development, drug prices, Research
STAT+: Pharmalittle: We’re reading about NIH removing scientific advisers, GSK’s shingles shot, and more
7 months 5 days ago
Pharma, Pharmalot, pharmalittle, STAT+
STAT+: Pharmalittle: We’re reading about a new CVS chief, obesity meds cutting overdoses, and more
1 year 1 week ago
Pharma, Pharmalot, pharmalittle, STAT+
STAT+: Pharmalittle: We’re reading about a Blue Cross California deal for Humira, Gilead licensing an HIV drug, and more
1 year 3 weeks ago
Pharma, Pharmalot, pharmalittle, STAT+